CBB1007LSD1 inhibitor,potent and selective CAS# 1379573-92-8 |
- CI994 (Tacedinaline)
Catalog No.:BCC2159
CAS No.:112522-64-2
- Tubastatin A HCl
Catalog No.:BCC3877
CAS No.:1310693-92-5
- M344
Catalog No.:BCC2162
CAS No.:251456-60-7
- AR-42 (OSU-HDAC42)
Catalog No.:BCC2161
CAS No.:935881-37-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1379573-92-8 | SDF | Download SDF |
| PubChem ID | 72199289 | Appearance | Powder |
| Formula | C27H34N8O4 | M.Wt | 534.61 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO | ||
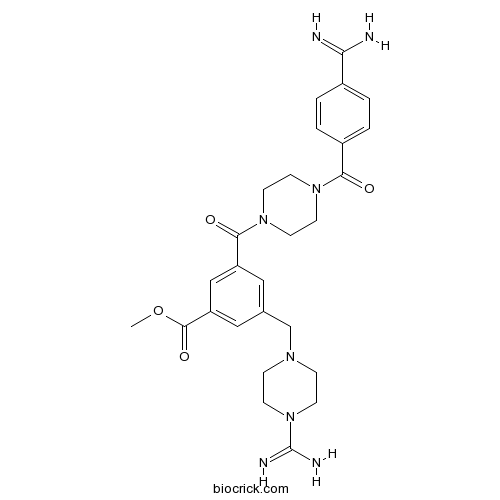
| Chemical Name | methyl 3-[4-(4-carbamimidoylbenzoyl)piperazine-1-carbonyl]-5-[(4-carbamimidoylpiperazin-1-yl)methyl]benzoate | ||
| SMILES | COC(=O)C1=CC(=CC(=C1)C(=O)N2CCN(CC2)C(=O)C3=CC=C(C=C3)C(=N)N)CN4CCN(CC4)C(=N)N | ||
| Standard InChIKey | IBVRETRIDAQSEM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C27H34N8O4/c1-39-26(38)22-15-18(17-32-6-8-35(9-7-32)27(30)31)14-21(16-22)25(37)34-12-10-33(11-13-34)24(36)20-4-2-19(3-5-20)23(28)29/h2-5,14-16H,6-13,17H2,1H3,(H3,28,29)(H3,30,31) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

CBB1007 Dilution Calculator

CBB1007 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8705 mL | 9.3526 mL | 18.7052 mL | 37.4104 mL | 46.7631 mL |
| 5 mM | 0.3741 mL | 1.8705 mL | 3.741 mL | 7.4821 mL | 9.3526 mL |
| 10 mM | 0.1871 mL | 0.9353 mL | 1.8705 mL | 3.741 mL | 4.6763 mL |
| 50 mM | 0.0374 mL | 0.1871 mL | 0.3741 mL | 0.7482 mL | 0.9353 mL |
| 100 mM | 0.0187 mL | 0.0935 mL | 0.1871 mL | 0.3741 mL | 0.4676 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
CBB1007 is an inhibitor of histone demethylase LSD1with IC50 values of 5.27μM [1].
LSD1 is a member of the FAD-dependent amine oxidase family. It converts di-methylated H3K4 to mono- and un-methylated H3K4 and suppresses gene expression. As an inhibitor of LSD1, CBB1007 is developed to understand the function of the enzyme. In the in vitro assay, CBB1007 shows highly potent inhibitory activity with IC50 value of 5.27μM. It is specific against LSD1 and has no effect on other histone demethylases including LSD2 and JARID1A. In the mouse F9 embryonic teratocarcinoma cells, CBB1007 cause a reproducible increase of mono- and di-methylated H3K4 with IC50 value of 1μM-5μM. It also activates the expression of CHRM4 and SCN3A which are the target genes of LSD1. The inhibition of LSD1 results in the growth inhibition of these cells. Furthermore, CBB1007 is found to inhibit the proliferation of other pluripotent cancer cells as well as the embryonic stem cells that express the stem cell markers Oct4 and Sox2 [1].
References:
[1] Wang J, Lu F, Ren Q, et al. Novel histone demethylase LSD1 inhibitors selectively target cancer cells with pluripotent stem cell properties. Cancer research, 2011, 71(23): 7238-7249.
- CBB1003
Catalog No.:BCC5524
CAS No.:1379573-88-2
- Arillatose B
Catalog No.:BCN6196
CAS No.:137941-45-8
- ML 239
Catalog No.:BCC3987
CAS No.:1378872-36-6
- 6-O-Feruloylglucose
Catalog No.:BCN6195
CAS No.:137887-25-3
- Valsartan methyl ester
Catalog No.:BCC9189
CAS No.:137863-17-3
- Valsartan
Catalog No.:BCC5017
CAS No.:137862-53-4
- 2,3-Di(3',4'-methylenedioxybenzyl)-2-buten-4-olide
Catalog No.:BCN1576
CAS No.:137809-97-3
- Boeravinone E
Catalog No.:BCN4083
CAS No.:137787-00-9
- 12-O-Acetylrosmarinine
Catalog No.:BCN2125
CAS No.:137760-53-3
- 7-Methoxy-beta-carboline-1-propionic acid
Catalog No.:BCN2995
CAS No.:137756-13-9
- 9-Methoxycanthin-6-one-N-oxide
Catalog No.:BCN2994
CAS No.:137739-74-3
- BIM 187
Catalog No.:BCC5933
CAS No.:137734-88-4
- Dehydrotolvaptan
Catalog No.:BCC8932
CAS No.:137973-76-3
- Mozavaptan
Catalog No.:BCC5095
CAS No.:137975-06-5
- Atroscine
Catalog No.:BCN1941
CAS No.:138-12-5
- Mafenide
Catalog No.:BCC5237
CAS No.:138-39-6
- D-(-)-Salicin
Catalog No.:BCN6298
CAS No.:138-52-3
- Picrocrocine
Catalog No.:BCC8232
CAS No.:138-55-6
- Shikimic acid
Catalog No.:BCN6200
CAS No.:138-59-0
- Limonene
Catalog No.:BCN3797
CAS No.:138-86-3
- Decorticasine
Catalog No.:BCN2006
CAS No.:1380-03-6
- BET bromodomain inhibitor
Catalog No.:BCC6426
CAS No.:1380087-89-7
- KB SRC 4
Catalog No.:BCC6253
CAS No.:1380088-03-8
- EPZ5676
Catalog No.:BCC2215
CAS No.:1380288-87-8
Inhibition of Lysine-Specific Demethylase-1 (LSD1/KDM1A) Promotes the Adipogenic Differentiation of hESCs Through H3K4 Methylation.[Pubmed:27059868]
Stem Cell Rev. 2016 Jun;12(3):298-304.
Given their totipotency, human embryonic stem cells (hESCs) can differentiate into all types of cells, including adipocytes, and provide an excellent research model for studying diseases associated with the metabolism of adipocytes, such as obesity and diabetes mellitus. Epigenetic regulation, including DNA methylation and histone modification, plays an essential role in the development and differentiation of hESCs. Lysine-specific demethylase 1 (LSD1), a well-characterized histone-modifying enzyme, demethylates dimethylated histone H3 lysine 4 (H3K4) through a flavin adenine dinucleotide (FAD)-dependent oxidative reaction. LSD1 affects the growth and differentiation of human and mouse ES cells, and the deletion of this gene in mice leads to embryonic lethality. Here, we investigated the functional role of LSD1 during the adipogenic differentiation of hESCs involving the demethylation of H3K4. We also found that treating hESCs with the LSD1 inhibitor CBB1007 promotes the adipogenic differentiation of hESCs.
Evaluation of Epigenetic Drug Targeting of Heterogenous Tumor Cell Fractions Using Potential Biomarkers of Response in Ovarian Cancer.[Pubmed:26130461]
Clin Cancer Res. 2015 Nov 15;21(22):5151-63.
PURPOSE: Resolution of aberrant epigenetic changes leading to altered gene expression during transformation and tumor progression is pertinent for mechanistic understanding of disrupted pathways in cancer. Such changes provide for biomarkers that can be applied in drug screening and improved disease management. EXPERIMENTAL DESIGN: Genome-wide profiling and analyses of promoter DNA methylation, histone modifications, and gene expression of an in vitro progression model of serous ovarian adenocarcinoma were carried out. Similar in silico analyses and comparison of methylation and gene expression of early- and late-grade ovarian cancer samples in The Cancer Genome Atlas assigned a clinical relevance to our study. Candidate biomarkers were evaluated for epigenetic drug treatments in experimental animal models on a background of differing tumor cell responses arising from intratumor heterogeneity. RESULTS: Differentially regulated genes during tumor progression were identified through the previously mentioned analyses as candidate biomarkers. In examining the tumor suppressor PTGIS as a potential biomarker for treatment with either 5-Aza-dC or TSA, 5-Aza-dC effectively stabilized cell cycling, restricted genetic instability, and derepressed PTGIS expression, while TSA led to emergence of drug-resistant progenitors lacking PTGIS expression. Profiling MEST and RXRgamma for curcumin and CBB1007, respectively, indicated an inability of curcumin and CBB1007 in restricting residual tumor regenerative capabilities. CONCLUSIONS: Our study provides novel insights into epigenetic regulation in ovarian cancer progression and potential biomarkers for evaluating efficacy of epigenetic drugs in restricting residual tumor regeneration. Such approaches may assign a new functional interpretation of drug efficacy and cell tumor responses in ovarian cancer.
Lysine-specific demethylase 1 inhibitors protect cochlear spiral ganglion neurons against cisplatin-induced damage.[Pubmed:26011390]
Neuroreport. 2015 Jun 17;26(9):539-47.
Cisplatin is a widely used chemotherapeutic drug, but one of its side effects is ototoxicity. Epigenetic-related drugs, such as lysine-specific demethylase 1 (LSD1) inhibitors, have been reported to protect against cisplatin-induced hair cell loss by preventing demethylation of histone H3K4 (H3K4me2). However, the protective effect of LSD1 inhibitors in spiral ganglion neurons (SGNs) remains unclear. To investigate whether LSD1 inhibitors exert similar protective effects on SGNs, we treated mouse cochlear explant cultures with LSD1 inhibitors (2PCPA, S2101, or CBB1007) together with cisplatin. Low concentrations of cisplatin damaged SGNs much more than high concentrations, and blocking the demethylation of H3K4me2 with LSD1 inhibitors prevented the SGNs from injury. Reactive oxygen species are also involved in the injury process, and LSD1 inhibitors protected SGNs by increasing the expression level of the antioxidant gene Slc7a11 and decreasing the level of the pro-oxidant gene lactoperoxidase (Lpo). Our findings show that LSD1 inhibitors prevent cisplatin-induced SGN loss by regulating the demethylation of H3K4 and preventing increases of reactive oxygen species levels, which might provide a potential therapeutic strategy for cisplatin-induced hearing loss.
Analysis of the levels of lysine-specific demethylase 1 (LSD1) mRNA in human ovarian tumors and the effects of chemical LSD1 inhibitors in ovarian cancer cell lines.[Pubmed:24165091]
J Ovarian Res. 2013 Oct 29;6(1):75.
BACKGROUND: Lysine-specific demethylase 1 (LSD1, also known as KDM1A and AOF2) is a chromatin-modifying activity that catalyzes the removal of methyl groups from lysine residues in histone and non-histone proteins, regulating gene transcription. LSD1 is overexpressed in several cancer types, and chemical inhibition of the LSD1 activity has been proposed as a candidate cancer therapy. Here, we examine the levels of LSD1 mRNA in human ovarian tumors and the cytotoxicity of several chemical LSD1 inhibitors in a panel of ovarian cancer cell lines. METHODS: We measured LSD1 mRNA levels in a cohort of n = 177 normal and heterogeneous tumor specimens by quantitative real time-PCR (qRT-PCR). Tumors were classified by FIGO stage, FIGO grade, and histological subtypes. We tested the robustness of our analyses in an independent cohort of n = 573 serous tumor specimens (source: TCGA, based on microarray). Statistical analyses were based on Kruskal-Wallis/Dunn's and Mann Whitney tests. Changes in LSD1 mRNA levels were also correlated with transcriptomic alterations at genome-wide scale. Effects on cell viability (MTS/PMS assay) of six LSD1 inhibitors (pargyline, TCP, RN-1, S2101, CAS 927019-63-4, and CBB1007) were also evaluated in a panel of ovarian cancer cell lines (SKOV3, OVCAR3, A2780 and cisplatin-resistant A2780cis). RESULTS: We found moderate but consistent LSD1 mRNA overexpression in stage IIIC and high-grade ovarian tumors. LSD1 mRNA overexpression correlated with a transcriptomic signature of up-regulated genes involved in cell cycle and down-regulated genes involved in the immune/inflammatory response, a signature previously observed in aggressive tumors. In fact, some ovarian tumors showing high levels of LSD1 mRNA are associated with poor patient survival. Chemical LSD1 inhibition induced cytotoxicity in ovarian cancer lines, which roughly correlated with their reported LSD1 inhibitory potential (RN-1,S2101 >> pargyline,TCP). CONCLUSIONS: Our findings may suggest a role of LSD1 in the biology of some ovarian tumors. It is of special interest to find a correlation of LSD1 mRNA overexpression with a transcriptomic signature relevant to cancer. Our findings, therefore, prompt further investigation of the role of LSD1 in ovarian cancer, as well as the study of its enzymatic inhibition in animal models for potential therapeutic purposes in the context of this disease.


