ChelerythrinePKC antagonist CAS# 34316-15-9 |
- CX-4945 (Silmitasertib)
Catalog No.:BCC3693
CAS No.:1009820-21-6
- Zoledronic Acid
Catalog No.:BCC1067
CAS No.:118072-93-8
- Enzastaurin (LY317615)
Catalog No.:BCC1100
CAS No.:170364-57-5
- Staurosporine
Catalog No.:BCC3612
CAS No.:62996-74-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 34316-15-9 | SDF | Download SDF |
| PubChem ID | 2703 | Appearance | Yellow powder |
| Formula | C21H18NO4 | M.Wt | 348.4 |
| Type of Compound | Alkaloids | Storage | Desiccate at -20°C |
| Solubility | >19.2mg/mL in DMSO | ||
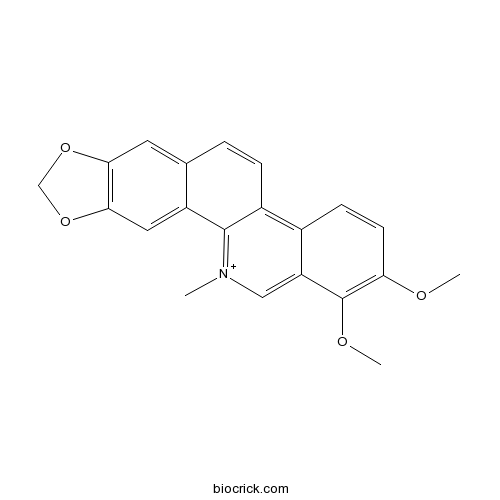
| Chemical Name | 1,2-dimethoxy-12-methyl-[1,3]benzodioxolo[5,6-c]phenanthridin-12-ium | ||
| SMILES | C[N+]1=C2C(=C3C=CC(=C(C3=C1)OC)OC)C=CC4=CC5=C(C=C42)OCO5 | ||
| Standard InChIKey | LLEJIEBFSOEYIV-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H18NO4/c1-22-10-16-13(6-7-17(23-2)21(16)24-3)14-5-4-12-8-18-19(26-11-25-18)9-15(12)20(14)22/h4-10H,11H2,1-3H3/q+1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Chelerythrine is a well-known protein kinase C inhibitor, can inhibit telomerase activity, it also can block the human P2X 7 receptor. Chelerythrine has antimanic, potential antiproliferative and antitumor effects, it has significant cytotoxic effect, independent of p53 and androgen status, on human prostate cancer cell lines. |
| Targets | PKC | cAMP | Bcl-2/Bax | p53 | Calcium Channel | ATPase | Sodium Channel | DNA/RNA Synthesis | HSP (e.g. HSP90) | p21 |
| In vitro | Chelerythrine induces reactive oxygen species-dependent mitochondrial apoptotic pathway in a murine T cell lymphoma.[Pubmed: 23900672]Tumour Biol. 2014 Jan;35(1):129-40.Chelerythrine is a well-known protein kinase C inhibitor and potential antiproliferative and antitumor pharmacological agent. Chelerythrine inhibits/suppresses the HSF1 phosphorylation by inhibiting PKC and blocks the nuclear migration and subsequent synthesis of hsp70 leading to reduced cell viability and activation of apoptotic machinery. Chelerythrine is also known to enhance the production of reactive oxygen intermediate that is strong activator of apoptosis in high concentration.
|
| In vivo | Partial effects of the protein kinase C inhibitor chelerythrine in a battery of tests for manic-like behavior in black Swiss mice.[Pubmed: 24948079]Pharmacol Rep. 2014 Aug;66(4):722-5.The aim of the present study was to test the effects of peripheral (intraperitoneal) administration of Chelerythrine in a battery of mania-related behavioral tests in black Swiss mice, a strain specific battery that was previously demonstrated to distinguish differential effects of mood stabilizing drugs. RESULTS: Sub-chronic administration of 1.0mg/kg or 2.0mg/kg Chelerythrine had marginal effects to reduce spontaneous activity and sweet solution preference in black Swiss mice which naturally show mania-like behaviors. Chelerythrine had no effects on the behavior of these mice in the elevated plus-maze, the forced swim test and the amphetamine-induced hyperactivity test. CONCLUSIONS: The partial effects in the battery are not unique as previous studies showed that lithium, valproate and risperidone, all used in the treatment of bipolar disorder, have distinct profiles in the battery. It is therefore concluded that Chelerythrine may have antimanic effects and additional dose and time response studies are warranted to further evaluate its range of activity. |
| Kinase Assay | Chelerythrine and other benzophenanthridine alkaloids block the human P2X7 receptor.[Pubmed: 15210579]Chelerythrine inhibits the sarco/endoplasmic reticulum Ca(2+)-ATPase and results in cell Ca(2+) imbalance.[Pubmed: 25721495]Arch Biochem Biophys. 2015 Mar 15;570:58-65.The isoquinoline alkaloid Chelerythrine is described as an inhibitor of SERCA.
Br J Pharmacol. 2004 Jul;142(6):1015-9.1 Extracellular ATP can activate a cation-selective channel/pore on human B-lymphocytes, known as the P2X7 receptor. Activation of this receptor is linked to PLD stimulation.
|
| Cell Research | The effect of chelerythrine on cell growth, apoptosis, and cell cycle in human normal and cancer cells in comparison with sanguinarine.[Pubmed: 16964588 ]Cell Biol Toxicol. 2006 Nov;22(6):439-53.
|
| Structure Identification | Biochemistry. 2015 Feb 3;54(4):974-86.Plant alkaloid chelerythrine induced aggregation of human telomere sequence--a unique mode of association between a small molecule and a quadruplex.[Pubmed: 25566806]
Small molecules that interact with G-quadruplex structures formed by the human telomeric region and stabilize them have the potential to evolve as anticancer therapeutic agents.
|

Chelerythrine Dilution Calculator

Chelerythrine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8703 mL | 14.3513 mL | 28.7026 mL | 57.4053 mL | 71.7566 mL |
| 5 mM | 0.5741 mL | 2.8703 mL | 5.7405 mL | 11.4811 mL | 14.3513 mL |
| 10 mM | 0.287 mL | 1.4351 mL | 2.8703 mL | 5.7405 mL | 7.1757 mL |
| 50 mM | 0.0574 mL | 0.287 mL | 0.5741 mL | 1.1481 mL | 1.4351 mL |
| 100 mM | 0.0287 mL | 0.1435 mL | 0.287 mL | 0.5741 mL | 0.7176 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Chelerythrine is a potent, selective antagonist of PKC (protein kinase C) with IC50 value of 0.66 μM.[1]
The alkaloid chelerythrine is a highly specific inhibitor that acts at the regulatory domain of the kinase.[2] It is also a competitive inhibitor with respect to the phosphate acceptor and a non-competitive inhibitor with respect to ATP.[1] Chelerythrine induced a dose-dependent decrease in the cell viability with IC50 value of 2.6 μM measured by MTT reduction assay.[3] Chelerythrine is also a selective and strong inhibitor of Bcl-xL functions and induced cell death in MEF cells with IC50 value of 1.1 μM.[4] Chelerythrine activated MEKK1- and MKK4-dependent JNK1 and p38 pathways then mediated the induction of apoptosis.[5] Chelerythrine stimulated apoptosis in the in vivo rat experiments (5 mg/kg) by inducing the generation of reactive oxygen species.[6] Chelerythrine also has widespread physiological effects on primarily antimicrobial and anti-inflammatory.
References:
1. J. M. Herbert, J. M. Augereau, J. Gleye and J. P. Maffrand, Biochem Biophys Res Commun 1990, 172, 993-999.
2. W. D. Jarvis, A. J. Turner, L. F. Povirk, R. S. Traylor and S. Grant, Cancer Res 1994, 54, 1707-1714.
3. J. Vrba, P. Dolezel, J. Vicar, M. Modriansky and J. Ulrichova, Toxicol In Vitro 2008, 22, 1008-1017.
4. M. Vogler, K. Weber, D. Dinsdale, I. Schmitz, K. Schulze-Osthoff, M. J. Dyer and G. M. Cohen, Cell Death Differ 2009, 16, 1030-1039.
5. R. Yu, S. Mandlekar, T. H. Tan and A. N. Kong, J Biol Chem 2000, 275, 9612-9619.
6. S. Yamamoto, K. Seta, C. Morisco, S. F. Vatner and J. Sadoshima, J Mol Cell Cardiol 2001, 33, 1829-1848.
- Boc-Abu-OH.DCHA
Catalog No.:BCC3200
CAS No.:27494-48-0
- 2,16-Kauranediol
Catalog No.:BCN5274
CAS No.:34302-37-9
- Harmine hydrochloride
Catalog No.:BCN2485
CAS No.:343-27-1
- 5,6-Dimethoxy-2-isopropenylbenzofuran
Catalog No.:BCN7195
CAS No.:34293-09-9
- 2,3-Dihydrohinokiflavone
Catalog No.:BCN6680
CAS No.:34292-87-0
- Chikusetsusaponin V methyl ester
Catalog No.:BCN3472
CAS No.:34291-22-0
- SDM25N hydrochloride
Catalog No.:BCC7054
CAS No.:342884-71-3
- Urotensin II-related peptide
Catalog No.:BCC5884
CAS No.:342878-90-4
- Gardneramine
Catalog No.:BCN5273
CAS No.:34274-91-4
- Angiotensin 1/2 (1-9)
Catalog No.:BCC1005
CAS No.:34273-12-6
- Saralasin
Catalog No.:BCC5714
CAS No.:34273-10-4
- Telbivudine
Catalog No.:BCC3862
CAS No.:3424-98-4
- TCS PrP Inhibitor 13
Catalog No.:BCC5999
CAS No.:34320-83-7
- OXA (17-33)
Catalog No.:BCC6364
CAS No.:343268-91-7
- Benzyl 2,6-dimethoxybenzoate
Catalog No.:BCN3697
CAS No.:34328-54-6
- Cirsiliol
Catalog No.:BCN6822
CAS No.:34334-69-5
- NSC697923
Catalog No.:BCC4000
CAS No.:343351-67-7
- NS 3623
Catalog No.:BCC6190
CAS No.:343630-41-1
- Isoapetalic acid
Catalog No.:BCN5276
CAS No.:34366-34-2
- Ginsenoside Ro
Catalog No.:BCN5937
CAS No.:34367-04-9
- CP-673451
Catalog No.:BCC4981
CAS No.:343787-29-1
- Acebutolol HCl
Catalog No.:BCC4322
CAS No.:34381-68-5
- Isovallesiachotamine
Catalog No.:BCN3549
CAS No.:34384-71-9
- N-Demethyl-alpha-obscurine
Catalog No.:BCN7362
CAS No.:34399-44-5
Plant alkaloid chelerythrine induced aggregation of human telomere sequence--a unique mode of association between a small molecule and a quadruplex.[Pubmed:25566806]
Biochemistry. 2015 Feb 3;54(4):974-86.
Small molecules that interact with G-quadruplex structures formed by the human telomeric region and stabilize them have the potential to evolve as anticancer therapeutic agents. Herein we report the interaction of a putative anticancer agent from a plant source, Chelerythrine, with the human telomeric DNA sequence. It has telomerase inhibitory potential as demonstrated from telomerase repeat amplification assay in cancer cell line extract. We have attributed this to the quadruplex binding potential of the molecule and characterized the molecular details of the interaction by means of optical spectroscopy such as absorbance and circular dichroism and calorimetric techniques such as isothermal titration calorimetry and differential scanning calorimetry. The results show that Chelerythrine binds with micromolar dissociation constant and 2:1 binding stoichiometry to the human telomeric DNA sequence. Chelerythrine association stabilizes the G-quadruplex. Nuclear magnetic resonance spectroscopy ((1)H and (31)P) shows that Chelerythrine binds to both G-quartet and phosphate backbone of the quadruplex leading to quadruplex aggregation. Molecular dynamics simulation studies support the above inferences and provide further insight into the mechanism of ligand binding. The specificity toward quartet binding for Chelerythrine is higher compared to that of groove binding. MM-PBSA calculation mines out the energy penalty for quartet binding to be -4.7 kcal/mol, whereas that of the groove binding is -1.7 kcal/mol. We propose that the first Chelerythrine molecule binds to the quartet followed by a second molecule which binds to the groove. This second molecule might bring about aggregation of the quadruplex structure which is evident from the results of nuclear magnetic resonance.
Chelerythrine and other benzophenanthridine alkaloids block the human P2X7 receptor.[Pubmed:15210579]
Br J Pharmacol. 2004 Jul;142(6):1015-9.
1 Extracellular ATP can activate a cation-selective channel/pore on human B-lymphocytes, known as the P2X7 receptor. Activation of this receptor is linked to PLD stimulation. We have used ATP-induced 86Rb+ (K+) efflux to examine the effect of benzophenanthridine alkaloids on P2X7 channel/pore function in human B-lymphocytes. 2 Both ATP and the nucleotide analogue 2'-3'-O-(4-benzoylbenzoyl)-ATP (BzATP) induced an 86Rb+ efflux, which was completely inhibited by the isoquinoline derivative 1-(N,O-bis[5-isoquinolinesulphonyl]-N-methyl-l-tyrosyl)-4-phenylpiperazine (KN-62), a potent P2X7 receptor antagonist. 3 The benzophenanthridine alkaloid Chelerythrine, a potent PKC inhibitor, inhibited the ATP-induced 86Rb+ efflux by 73.4+/-3.5% and with an IC50 of 5.6+/-2.3 microm. Similarly, other members of this family of compounds, sanguinarine and berberine, blocked the ATP-induced 86Rb+ efflux by 58.8+/-4.8 and 61.1+/-8.0%, respectively. 4 Concentration-effect curves to ATP estimated an EC50 value of 78 microm and in the presence of 5 and 10 microm Chelerythrine this increased slightly to 110 and 150 microm, respectively, which fits a noncompetitive inhibitor profile for Chelerythrine. 5 Chelerythrine at 10 microm was effective at inhibiting the ATP-induced PLD stimulation in B-lymphocytes by 94.2+/-21.9% and the phorbol 12-myristate 13-acetate-induced PLD stimulation by 68.2+/-7.4%. 6 This study demonstrates that Chelerythrine in addition to PKC inhibition has a noncompetitive inhibitory action on the P2X7 receptor itself.
Chelerythrine induces reactive oxygen species-dependent mitochondrial apoptotic pathway in a murine T cell lymphoma.[Pubmed:23900672]
Tumour Biol. 2014 Jan;35(1):129-40.
Chelerythrine is a well-known protein kinase C inhibitor and potential antiproliferative and antitumor pharmacological agent. Chelerythrine inhibits/suppresses the HSF1 phosphorylation by inhibiting PKC and blocks the nuclear migration and subsequent synthesis of hsp70 leading to reduced cell viability and activation of apoptotic machinery. Chelerythrine is also known to enhance the production of reactive oxygen intermediate that is strong activator of apoptosis in high concentration. Therefore, the present study intended to investigate the role of Chelerythrine-induced reactive oxygen intermediate on the viability and apoptosis of Dalton's lymphoma cells. Enhanced production of reactive oxygen species in Dalton's lymphoma (DL) cells was observed upon treatment of Chelerythrine only which was seen completely abolished on treatment of mitochondrial complex inhibitors rotenone and malonate, and anti-oxidant, N-acetyl-L-cysteine. Increased number of DL cells undergoing apoptosis, as observed by fluorescent microscopy and flow cytometry analysis, in Chelerythrine only-treated group was seen that was significantly inhibited on treatment of mitochondrial complex inhibitors and anti-oxidants. Staurosporine, on the other hand, does not lead to enhanced production of reactive oxygen intermediate in DL cells.
The effect of chelerythrine on cell growth, apoptosis, and cell cycle in human normal and cancer cells in comparison with sanguinarine.[Pubmed:16964588]
Cell Biol Toxicol. 2006 Nov;22(6):439-53.
We compared the effects of Chelerythrine (CHE) and sanguinarine (SA) on human prostate cancer cell lines (LNCaP and DU-145) and primary culture of human gingival fibroblasts. CHE and SA treatment of cell lines for 24 h resulted in (1) inhibition of cell viability in a dose-dependent manner in all tested cells (as evaluated by MTT test and bromodeoxyuridine incorporation assay); (2) dose-dependent increase in DNA damage in all tested cells (as evaluated by DNA comet assay); (3) changes in apoptosis (assessed by western blot analysis and TUNEL assay); and (4) significant induction of cyclin kinase inhibitors p21(Waf1/Cip1) and p27(Kip1) in prostate cancer cells (identified by western blot analysis). Our study demonstrates that CHE had significant cytotoxic effect, independent of p53 and androgen status, on human prostate cancer cell lines. Normal gingival fibroblasts and DU-145 cells were more sensitive to the treatment with both alkaloids than were LNCaP cells. CHE and SA may be prospective natural molecules for use in the treatment of prostate cancer owing to their involvement in apoptosis and cell cycle regulation.
Partial effects of the protein kinase C inhibitor chelerythrine in a battery of tests for manic-like behavior in black Swiss mice.[Pubmed:24948079]
Pharmacol Rep. 2014 Aug;66(4):722-5.
BACKGROUND: The inhibition of protein kinase C (PKC) was recently suggested as a novel approach for the development of mood stabilizing drugs. METHODS: To further evaluate this possibility, the aim of the present study was to test the effects of peripheral (intraperitoneal) administration of Chelerythrine in a battery of mania-related behavioral tests in black Swiss mice, a strain specific battery that was previously demonstrated to distinguish differential effects of mood stabilizing drugs. RESULTS: Sub-chronic administration of 1.0mg/kg or 2.0mg/kg Chelerythrine had marginal effects to reduce spontaneous activity and sweet solution preference in black Swiss mice which naturally show mania-like behaviors. Chelerythrine had no effects on the behavior of these mice in the elevated plus-maze, the forced swim test and the amphetamine-induced hyperactivity test. CONCLUSIONS: The partial effects in the battery are not unique as previous studies showed that lithium, valproate and risperidone, all used in the treatment of bipolar disorder, have distinct profiles in the battery. It is therefore concluded that Chelerythrine may have antimanic effects and additional dose and time response studies are warranted to further evaluate its range of activity.
Recognition of chelerythrine to human telomeric DNA and RNA G-quadruplexes.[Pubmed:25341562]
Sci Rep. 2014 Oct 24;4:6767.
A study on binding of antitumor Chelerythrine to human telomeric DNA/RNA G-quadruplexes was performed by using DNA polymerase stop assay, UV-melting, ESI-TOF-MS, UV-Vis absorption spectrophotometry and fluorescent triazole orange displacement assay. Chelerythrine selectively binds to and stabilizes the K(+)-form hybrid-type human telomeric DNA G-quadruplex of biological significance, compared with the Na(+)-form antiparallel-type DNA G-quadruplex. ESI-TOF-MS study showed that Chelerythrine possesses a binding strength for DNA G-quadruplex comparable to that of TMPyP4 tetrachloride. Both 1:1 and 2:1 stoichiometries were observed for Chelerythrine's binding with DNA and RNA G-quadruplexes. The binding strength of Chelerythrine with RNA G-quadruplex is stronger than that with DNA G-quadruplex. Fluorescent triazole orange displacement assay revealed that Chelerythrine interacts with human telomeric RNA/DNA G-quadruplexes by the mode of end- stacking. The relative binding strength of Chelerythrine for human telomeric RNA and DNA G-quadruplexes obtained from ESI-TOF-MS experiments are respectively 6.0- and 2.5-fold tighter than that with human telomeric double-stranded hairpin DNA. The binding selectivity of Chelerythrine for the biologically significant K(+)-form human telomeric DNA G-quadruplex over the Na(+)-form analogue, and binding specificity for human telomeric RNA G-quadruplex established it as a promising candidate in the structure-based design and development of G-quadruplex specific ligands.
Chelerythrine inhibits the sarco/endoplasmic reticulum Ca(2+)-ATPase and results in cell Ca(2+) imbalance.[Pubmed:25721495]
Arch Biochem Biophys. 2015 Mar 15;570:58-65.
The isoquinoline alkaloid Chelerythrine is described as an inhibitor of SERCA. The ATPase inhibition presented two non-competitive components, Ki1=1, 2 muM and Ki2=26 muM. Conversely, Chelerythrine presented a dual effect on the p-nitrophenylphosphatase (pNPPase) of SERCA. Ca(2+)-dependent pNPPase was activated up to approximately 5 muM Chelerythrine with inhibition thereafter. Ca(2+)-independent pNPPase was solely inhibited. The phosphorylation of SERCA with ATP reached half-inhibition with 10 muM Chelerythrine and did not parallel the decrease of ATPase activity. In contrast, Chelerythrine up to 50 muM increased the phosphorylation by Pi. Cross-linking of SERCA with glutaraldehyde was counteracted by high concentrations of Chelerythrine. The controlled tryptic digestion of SERCA shows that the low-affinity binding of Chelerythrine evoked an E2-like pattern. Our data indicate a non-competitive inhibition of ATP hydrolysis that favors buildup of the E2-conformers of the enzyme. Chelerythrine as low as 0.5-1.5 muM resulted in an increase of intracellular Ca(2+) on cultured PBMC cells. The inhibition of SERCA and the loss of cell Ca(2+) homeostasis could in part be responsible for some described cytotoxic effects of the alkaloid. Thus, the choice of Chelerythrine as a PKC-inhibitor should consider its potential cytotoxicity due to the alkaloid's effects on SERCA.


