Cinnamic acidCAS# 140-10-3 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 140-10-3 | SDF | Download SDF |
| PubChem ID | 444539 | Appearance | White-beige powder |
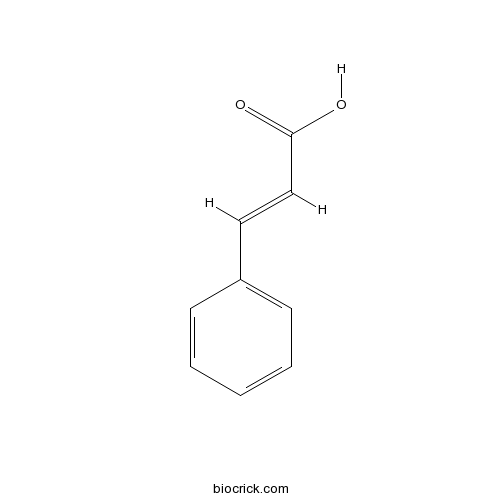
| Formula | C9H8O2 | M.Wt | 148.2 |
| Type of Compound | Phenylpropanoids | Storage | Desiccate at -20°C |
| Synonyms | 621-82-9;TRANS-CINNAMIC ACID;(E)-Cinnamic Acid; Trans-3-Phenylacrylic Acid; 3-Phenylacrylic Acid; Phenylacrylic Acid | ||
| Solubility | >7.3mg/mL in DMSO | ||
| Chemical Name | (E)-3-phenylprop-2-enoic acid | ||
| SMILES | C1=CC=C(C=C1)C=CC(=O)O | ||
| Standard InChIKey | WBYWAXJHAXSJNI-VOTSOKGWSA-N | ||
| Standard InChI | InChI=1S/C9H8O2/c10-9(11)7-6-8-4-2-1-3-5-8/h1-7H,(H,10,11)/b7-6+ | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Cinnamic acid, a naturally occurring aromatic fatty acid of low toxicity, has anti-diabetic , anticancer and antioxidant activities. It is a cell differentiation inducer and protein isoprenylation inhibitor, shows a significant radio-protective effect by reducing the DNA damage induced by X-rays. Cinnamic acid inhibited feeding by detritivores, this inhibition occurs at concentrations found in nature and may be a major factor controlling the rate of decay of organic matter. It inhibited mushroom tyrosinase activity in reversiblywith the IC 50 value of 2.10 mM. |
| Targets | p53 | c-myc | c-fos | Tyrosinase |
| In vitro | Radio-protective effect of cinnamic acid, a phenolic phytochemical, on genomic instability induced by X-rays in human blood lymphocytes in vitro.[Pubmed: 25344167]Mutat Res Genet Toxicol Environ Mutagen. 2014 Aug;770:72-9.
Incidence of Fusarium wilt in Cucumis sativus L. is promoted by cinnamic acid, an autotoxin in root exudates[Reference: WebLink]Plant & Soil, 2004, 263(1):143-50.
Cinnamic acid inhibition of detritus feeding. [Reference: WebLink]Nature, 1979, 280(280):55-7.AN important advance in ecology has been the general acceptance of Fraenkel's postulate that certain chemicals in plants deter herbivores1–3. Such chemicals are usually termed secondary plant substances because they are not involved in primary metabolic pathways. Very little of the annual production of biomass by higher plants is consumed by herbivores or phyto-pathogens4. Instead, most of the biomass becomes litter and eventually decays through the activity of decomposers. The secondary compounds that deter grazers while the plants are alive do not disappear immediately when plants senesce and die. We have therefore investigated whether these anti-herbivore substances continue to inhibit consumption by organisms feeding on litter or detritus. We report here that Cinnamic acids, one type of secondary plant substances found in detritus, inhibit feeding by detritivores. This inhibition occurs at concentrations found in nature and may be a major factor controlling the rate of decay of organic matter. |
| In vivo | Cinnamic acid exerts anti-diabetic activity by improving glucose tolerance in vivo and by stimulating insulin secretion in vitro.[Pubmed: 25765836]Phytomedicine. 2015 Feb 15;22(2):297-300.Although the anti-diabetic activity of Cinnamic acid, a pure compound from cinnamon, has been reported but its mechanism(s) is not yet clear.
|
| Kinase Assay | Inhibitory effects of cinnamic acid and its derivatives on the diphenolase activity of mushroom (Agaricus bisporus) tyrosinase[Reference: WebLink]Anticancer effects of ginsenoside Rg1, cinnamic acid, and tanshinone IIA in osteosarcoma MG-63 cells: nuclear matrix downregulation and cytoplasmic trafficking of nucleophosmin.[Pubmed: 18403247]Int J Biochem Cell Biol. 2008;40(9):1918-29.Ginsenoside Rg1, Cinnamic acid, and tanshinone IIA are effective anticancer and antioxidant constituents of traditional Chinese herbal medicines of Ginseng (Panax ginseng), Xuanshen (Radix scrophulariae), and Danshen (Salvia mitiorrhiza), respectively. There was insufficient study on molecular mechanisms of anticancer effects of those constituents and their targets were unknown.
Food Chem., 2005, 92(4):707-12.The effects of Cinnamic acid and its derivatives (2-hydroxyCinnamic acid, 4-hydroxyCinnamic acid and 4-methoxyCinnamic acid) on the activity of mushroom tyrosinase have been studied. Results showed that Cinnamic acid, 4-hydroxyCinnamic acid and 4-methoxyCinnamic acid strongly inhibited the diphenolase activity of mushroom tyrosinase and the inhibition was reversible. The IC50 values were estimated to be 2.10, 0.50 and 0.42 mM, respectively. 2-HydroxyCinnamic acid had no inhibitory effect on the diphenolase activity of the enzyme. Kinetic analyses showed that the inhibition type of Cinnamic acid and 4-methoxyCinnamic acid was noncompetitive with the constants (KI) determined to be 1.994 and 0.458 mM, respectively. The inhibition type of 4-hydroxyCinnamic acid was competitive, with the inhibition constant (KI) was 0.244 mM. |

Cinnamic acid Dilution Calculator

Cinnamic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.7476 mL | 33.7382 mL | 67.4764 mL | 134.9528 mL | 168.691 mL |
| 5 mM | 1.3495 mL | 6.7476 mL | 13.4953 mL | 26.9906 mL | 33.7382 mL |
| 10 mM | 0.6748 mL | 3.3738 mL | 6.7476 mL | 13.4953 mL | 16.8691 mL |
| 50 mM | 0.135 mL | 0.6748 mL | 1.3495 mL | 2.6991 mL | 3.3738 mL |
| 100 mM | 0.0675 mL | 0.3374 mL | 0.6748 mL | 1.3495 mL | 1.6869 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Palbinone
Catalog No.:BCN3930
CAS No.:139954-00-0
- Alpinone 3-acetate
Catalog No.:BCN7768
CAS No.:139906-49-3
- Drahebenine
Catalog No.:BCN7044
CAS No.:1399049-43-4
- Globularin
Catalog No.:BCN6215
CAS No.:1399-49-1
- 3,6'-Disinapoyl sucrose
Catalog No.:BCN2719
CAS No.:139891-98-8
- Milameline hydrochloride
Catalog No.:BCC7427
CAS No.:139886-04-7
- Cucurbitacin E-2-O-Glucoside
Catalog No.:BCC8156
CAS No.:1398-78-3
- Sildenafil
Catalog No.:BCC1947
CAS No.:139755-83-2
- tenuifoliside C
Catalog No.:BCN8299
CAS No.:139726-37-7
- Tenuifoliside B
Catalog No.:BCC9251
CAS No.:139726-36-6
- Tenuifoliside A
Catalog No.:BCN2893
CAS No.:139726-35-5
- Isodunnianol
Catalog No.:BCN6213
CAS No.:139726-30-0
- Nithiamide
Catalog No.:BCC4687
CAS No.:140-40-9
- Pentamidine isethionate
Catalog No.:BCC5644
CAS No.:140-64-7
- 4-Allylanisole
Catalog No.:BCC8674
CAS No.:140-67-0
- Dimethylolurea
Catalog No.:BCC8943
CAS No.:140-95-4
- Nystatin (Fungicidin)
Catalog No.:BCC4813
CAS No.:1400-61-9
- 4μ8C
Catalog No.:BCC4754
CAS No.:14003-96-4
- Eucalyptin acetate
Catalog No.:BCN6216
CAS No.:14004-35-4
- PB-22
Catalog No.:BCC1840
CAS No.:1400742-17-7
- 3-CPMT
Catalog No.:BCC6845
CAS No.:14008-79-8
- Tannic acid
Catalog No.:BCN2643
CAS No.:1401-55-4
- AMG 925
Catalog No.:BCC5150
CAS No.:1401033-86-0
- 3'-Hydroxygynuramide II
Catalog No.:BCC8634
CAS No.:1401093-57-9
Mechanism of cinnamic acid-induced trypsin inhibition: a multi-technique approach.[Pubmed:23954540]
Spectrochim Acta A Mol Biomol Spectrosc. 2013 Dec;116:251-7.
In order to investigate the association of the protease trypsin with Cinnamic acid, the interaction was characterized by using fluorescence, UV-vis absorption spectroscopy, molecular modeling and an enzymatic inhibition assay. The binding process may be outlined as follows: Cinnamic acid can interact with trypsin with one binding site to form Cinnamic acid-trypsin complex, resulting in inhibition of trypsin activity; the spectroscopic data show that the interaction is a spontaneous process with the estimated enthalpy and entropy changes being -8.95 kJ mol(-1) and 50.70 J mol(-1) K(-1), respectively. Noncovalent interactions make the main contribution to stabilize the trypsin-Cinnamic acid complex; Cinnamic acid can enter into the primary substrate-binding pocket and alter the environment around Trp and Tyr residues.
Comparative phytotoxicity of usnic acid, salicylic acid, cinnamic acid and benzoic acid on photosynthetic apparatus of Chlamydomonas reinhardtii.[Pubmed:29751250]
Plant Physiol Biochem. 2018 Jul;128:1-12.
The effects of four phytotoxins usnic acid (UA), salicylic acid (SA), Cinnamic acid (CA) and benzoic acid (BA) on photosynthesis of Chlamydomonas reinhardtii were studied in vivo to identify and localise their initial action sites on two photosystems. Our experimental evidence shows that the four phytotoxins have multiple targets in chloroplasts, which mainly lie in photosystem II (PSII), not photosystem I (PSI). They share an original action site by blocking electron transport beyond QA (primary plastoquinone acceptor) at PSII acceptor side since a fast increase of the J-step level is the greatest change in chlorophyll a fluorescence induction kinetics OJIP in C. reinhardtii cells treated with the phytotoxins. UA decreases photosynthetic activity by reducing O2 evolution rate, interrupting PSII electron transport at both the donor and acceptor sides, inactivating the PSII reaction centers (RCs), reducing the content of chlorophylls and carotenoids, destroying the conformation of antenna pigment assemblies, and casuing the degradation of D1/D2 proteins. SA damage to photosynthetic machinery is mainly attributed to inhibition of PSII electron transport beyond QA at the acceptor side, inactivation of the PSII RCs, reduction of chlorophyll content, digestion of thylakoid ploypeptides and destabilization of thylakoid membranes. Both CA and BA affect the photosynthetic process by decreasing PSII electron transport efficiency at the acceptor side and the amount of active PSII RCs. Besides, the initial cause of BA-inhibiting photosynthesis is also assocaited with the O2 evolution rate and the disconnection of some antenna molecules from PSII RCs.
UHPLC-QqQ-MS/MS identification, quantification of polyphenols from Passiflora subpeltata fruit pulp and determination of nutritional, antioxidant, alpha-amylase and alpha-glucosidase key enzymes inhibition properties.[Pubmed:29735097]
Food Res Int. 2018 Jun;108:611-620.
The diabetic key enzymes inhibition, nutritional, antioxidant activity and bioactive compounds identification of Passiflora subpeltata fruit pulp were investigated. Fifteen polyphenolic compounds including protocatechuic acid, ferulic acid, vanillic acid, epicatechin, p-coumaric acid, Cinnamic acid, eriodictyol and quercetin-3-glucoside were identified in the pulp of this species by using UHPLC-QqQ-MS/MS analysis. The total carbohydrates and crude protein contents in fruit pulp were 2.62mg glucose equivalent/g sample fruit pulp and 8.80mg BSA equivalent/g sample fruit pulp, respectively. The fresh fruit pulp of P. subpeltata contained high total phenolic (724.76mg GAE/g sample) content and it revealed very high DPPH(*) (IC50 of 5.667mug/mL) and ABTS(+*) (6794.96muM trolox equivalent/g sample) scavenging activities. In the key enzymes assays useful for diabetic inhibition the fresh fruit pulp characterized maximum inhibition of alpha-amylase and alpha-glucosidase IC50 of 18.69 and 32.63mug/mL, respectively. Thus, these results lead to conclude that this fruit specie could be very useful source in nutraceutical products preparations for Type 2 diabetic suffering humans.
Cinnamic acid exerts anti-diabetic activity by improving glucose tolerance in vivo and by stimulating insulin secretion in vitro.[Pubmed:25765836]
Phytomedicine. 2015 Feb 15;22(2):297-300.
Although the anti-diabetic activity of Cinnamic acid, a pure compound from cinnamon, has been reported but its mechanism(s) is not yet clear. The present study was designed to explore the possible mechanism(s) of anti-diabetic activity of Cinnamic acid in in vitro and in vivo non-obese type 2 diabetic rats. Non-obese type 2 diabetes was developed by injecting 90 mg/kg streptozotocin in 2-day-old Wistar pups. Cinnamic acid and cinnamaldehyde were administered orally to diabetic rats for assessing acute blood glucose lowering effect and improvement of glucose tolerance. Additionally, insulin secretory activity of Cinnamic acid and cinnamaldehyde was evaluated in isolated mice islets. Cinnamic acid, but not cinnamaldehyde, decreased blood glucose levels in diabetic rats in a time- and dose-dependent manner. Oral administration of Cinnamic acid with 5 and 10 mg/kg doses to diabetic rats improved glucose tolerance in a dose-dependent manner. The improvement by 10 mg/kg Cinnamic acid was comparable to that of standard drug glibenclamide (5 mg/kg). Further in vitro studies showed that cinnamaldehyde has little or no effect on glucose-stimulated insulin secretion; however, Cinnamic acid significantly enhanced glucose-stimulated insulin secretion in isolated islets. In conclusion, it can be said that Cinnamic acid exerts anti-diabetic activity by improving glucose tolerance in vivo and stimulating insulin secretion in vitro.
Repellence of Common Tobacco Flavorants on Lasioderma serricorne (Coleoptera: Anobiidae).[Pubmed:29762719]
J Econ Entomol. 2018 May 12. pii: 4995541.
The cigarette beetle, Lasioderma serricorne (F.) (Coleoptera: Anobiidae) is a destructive pest species of tobacco. Olfactory repellents derived from permitted tobacco flavorants have the advantage of not adversely effecting tobacco flavor. Among 12 test compounds, neral exhibited the strongest repellent effect. Among six binary blends prepared, three blends (neral + ethyl cinnamate, neral + cinnamaldehyde, and neral + methyl cinnamate) evoked the strongest repellent response. The interactions between neral and any one of the Cinnamic acid derivatives were additive, and the interactions between neral and the Cinnamic acid derivatives were antagonistic. In a 32-d tobacco barn bioassay, neral + cinnamaldehyde (embedded in 0.5% agaropectin) showed the strongest repellent effect with a persistence of at least 30 d. The binary blend of two tobacco additives (neral and cinnamaldehyde) appears promising as a repellent for controlling cigarette beetles in tobacco barns.
The allelochemical trans-cinnamic acid stimulates salicylic acid production and galactose pathway in maize leaves: A potential mechanism of stress tolerance.[Pubmed:29753136]
Plant Physiol Biochem. 2018 Jul;128:32-40.
In this study, the effects (5 days) of the secondary metabolite trans-Cinnamic acid on maize leaves (Zea mays L.), through a physiological and an untargeted metabolomic approach, were evaluated. A reduction in leaf growth and development accompanied by a decrease in protein content was observed in treated seedlings. Besides, trans-Cinnamic acid stimulated the photosynthetic machinery with a significant increment in pigment content (chlorophyll a, b and carotenoids), a stimulation of the light adapted PSII efficiency (II) as well as the chlorophyll a fluorescence (YNO), the apparent electron transport rate, and the regulated dissipation of the energy (YNPQ). By contrast, the dark adapted PSII parameter (Fv/Fm) was not affected suggesting that no physical damages to the antenna complex were caused by trans-Cinnamic acid. These results suggested that maize seedlings were experiencing a stress but, at the same time, were able to cope with it. This hypothesis was confirmed by both the increment in benzoic and salicylic acids, important molecules involved in stress response, and the metabolomic results, which pointed out that the seedlings are directing their metabolism towards galactose production modulating its pathway, which is pivotal for the production of the antioxidant compound ascorbic acid (ASA). Indeed, in treated plants, a significant increment in total ASA content (28%) was observed. The results suggested that the main strategy adopted by plants to cope with trans-cinnamic-induced stress consisted in the modulation of their metabolism in order to increase the total ASA and carotenoids concentration, radical scavenging species.
Phenolic compounds as beneficial phytochemicals in pomegranate (Punica granatum L.) peel: A review.[Pubmed:29739608]
Food Chem. 2018 Sep 30;261:75-86.
Pomegranate peel (PoP), a juice byproduct often considered as a waste, comprises nearly around 30-40% portion of the fruit. Phenolic compounds (one class of bioactive phytochemicals) are primarily concentrated in the peel portion of pomegranate fruit. In PoP, the main phenolic compounds reported in the literature include flavonoids (anthocyanins such as pelargonidin, delphinidin, cyanidin along with their derivatives and anthoxanthins such as catechin, epicatechin and quercetin), tannins (ellagitannins and ellagic acid derivatives such as punicalagin, punicalin and pedunculagin) and phenolic acids (such as chlorogenic, caffeic, syringic, sinapic, p-coumaric, ferulic, ellagic, gallic and Cinnamic acid). It is generally accepted that phenolic compounds can be more efficiently recovered from PoP by improving the extraction efficiency. The curative relevance of these compounds has been mainly assessed by in vitro experimentation. Therefore, conclusive clinical trials of the phenolic compounds present in PoP are essential for correct validation of their health benefits.
Radio-protective effect of cinnamic acid, a phenolic phytochemical, on genomic instability induced by X-rays in human blood lymphocytes in vitro.[Pubmed:25344167]
Mutat Res Genet Toxicol Environ Mutagen. 2014 Aug;770:72-9.
The present study was designed to determine the protective activity of Cinnamic acid against induction by X-rays of genomic instability in normal human blood lymphocytes. This radio-protective activity was assessed by use of the cytokinesis-block micronucleus test and the alkaline comet assay, with human blood lymphocytes isolated from two healthy donors. A Siemens Mevatron MD2 (Siemens AG, USA, 1994) linear accelerator was used for the irradiation with 1 or 2 Gy. Treatment of the lymphocytes with Cinnamic acid prior to irradiation reduced the number of micronuclei when compared with that in control samples. Treatment with Cinnamic acid without irradiation did not increase the number of micronuclei and did not show a cytostatic effect in the lymphocytes. The results of the alkaline comet assay revealed that Cinnamic acid reduces the DNA damage induced by X-rays, showing a significant radio-protective effect. Cinnamic acid decreased the frequency of irradiation-induced micronuclei by 16-55% and reduced DNA breakage by 17-50%, as determined by the alkaline comet assay. Cinnamic acid may thus act as a radio-protective compound, and future studies may focus on elucidating the mechanism by which Cinnamic acid offers radioprotection.
Anticancer effects of ginsenoside Rg1, cinnamic acid, and tanshinone IIA in osteosarcoma MG-63 cells: nuclear matrix downregulation and cytoplasmic trafficking of nucleophosmin.[Pubmed:18403247]
Int J Biochem Cell Biol. 2008;40(9):1918-29.
Ginsenoside Rg1, Cinnamic acid, and tanshinone IIA are effective anticancer and antioxidant constituents of traditional Chinese herbal medicines of Ginseng (Panax ginseng), Xuanshen (Radix scrophulariae), and Danshen (Salvia mitiorrhiza), respectively. There was insufficient study on molecular mechanisms of anticancer effects of those constituents and their targets were unknown. We chose nucleophosmin as a candidate molecular target because it is frequently mutated and upregulated in various cancer cells. Nucleophosmin is a major nucleolus phosphoprotein that involves in rRNA synthesis, maintaining genomic stability, and normal cell division and its haploinsufficiency makes cell more susceptible to oncogenic assault. Ginsenoside Rg1, Cinnamic acid, and tanshinone IIA treatment of osteosarcoma MG-63 cells decreased nucleophosmin expression in nuclear matrix and induced nucleophosmin translocation from nucleolus to nucleoplasm and cytoplasm, a process of dedifferentiating transformed cells. Using immunogold electro-microscopy, we found at the first time that nucleophosmin was localized on nuclear matrix intermediate filaments that had undergone restorational changes after the treatments. Nucleophosmin also functions as a molecular chaperone that might interact with multiple oncogenes and tumor suppressor genes. We found that oncogenes c-myc, c-fos and tumor suppressor genes, P53, Rb were regulated by ginsenoside Rg1, Cinnamic acid, and tanshinone IIA as well. In present study, we identified nucleophosmin as a molecular target of the effective anticancer constituents of t Ginseng, Xuanseng, and Danseng that down-regulated nucleophosmin in nuclear matrix, changed its trafficking from nucleolus to cytoplasm, and regulated several oncogenes and tumor suppressor genes. Therefore, we postulate that Ginsenoside Rg1, Cinnamic acid, and tanshinone IIA could serve as protective agents in cancer prevention and treatment.
Self-Assembled Ag-Cu(2)O Nanocomposite Films at Air-Liquid Interfaces for Surface-Enhanced Raman Scattering and Electrochemical Detection of H(2)O(2).[Pubmed:29762527]
Nanomaterials (Basel). 2018 May 15;8(5). pii: nano8050332.
We employ a facile and novel route to synthesize multifunctional Ag-Cu(2)O nanocomposite films through the self-assembly of nanoparticles at an air-liquid interface. In the ethanol-water phase, AgNO(3) and Cu(NO(3))(2) were reduced to Ag-Cu(2)O nanoparticles by NaBH(4) in the presence of Cinnamic acid. The Ag-Cu(2)O nanoparticles were immediately trapped at the air-liquid interface to form two-dimensional nanocomposite films after the reduction reaction was finished. The morphology of the nanocomposite films could be controlled by the systematic regulation of experimental parameters. It was found that the prepared nanocomposite films serving as the substrates exhibited strong surface-enhanced Raman scattering (SERS) activity. 4-aminothiophenol (4-ATP) molecules were used as the test probes to examine the SERS sensitivity of the nanocomposite films. Moreover, the nanocomposite films synthesized by our method showed enhanced electrocatalytic activity towards hydrogen peroxide (H(2)O(2)) and therefore could be utilized to fabricate a non-enzymatic electrochemical H(2)O(2) sensor.
Understanding Which Residues of the Active Site and Loop Structure of a Tyrosine Aminomutase Define Its Mutase and Lyase Activities.[Pubmed:29757631]
Biochemistry. 2018 Jun 26;57(25):3503-3514.
Site-directed mutations and substrate analogues were used to gain insights into the branch-point reaction of the 3,5-dihydro-5-methylidene-4 H-imidazol-4-one (MIO)-tyrosine aminomutase from Oryza sativa ( OsTAM). Exchanging the active residues of OsTAM (Y125C/N446K) for those in a phenylalanine aminomutase TcPAM altered its substrate specificity from tyrosine to phenylalanine. The aminomutase mechanism of OsTAM surprisingly changed almost exclusively to that of an ammonia lyase making Cinnamic acid (>95%) over beta-phenylalanine [Walter, T., et al. (2016) Biochemistry 55, 3497-3503]. We hypothesized that the missing electronics or sterics on the aryl ring of the phenylalanine substrate, compared with the sizable electron-donating hydroxyl of the natural tyrosine substrate, influenced the unexpected lyase reactivity of the OsTAM mutant. The double mutant was incubated with 16 alpha-phenylalanine substituent analogues of varying electronic strengths and sterics. The mutant converted each analogue principally to its acrylate with approximately 50% conversion of the p-Br substrate, making only a small amount of the beta-amino acid. The inner loop structure over the entrance to the active site was also mutated to assess how the lyase and mutase activities are affected. An OsTAM loop mutant, matching the loop residues of TcPAM, still chiefly made >95% of the acrylate from each substrate. A combined active site:loop mutant was most reactive but remained a lyase, making 10-fold more acrylates than other mutants did. While mutations within the active site changed the substrate specificity of OsTAM, continued exploration is needed to fully understand the interplay among the inner loop, the substrate, and the active site in defining the mutase and lyase activities.


