SildenafilPDE5 inhibitor, selective CAS# 139755-83-2 |
- GSK256066 2,2,2-trifluoroacetic acid
Catalog No.:BCC1605
CAS No.:1415560-64-3
- Nortadalafil
Catalog No.:BCC1806
CAS No.:171596-36-4
- Bay 60-7550
Catalog No.:BCC1405
CAS No.:439083-90-6
- Oglemilast
Catalog No.:BCC1817
CAS No.:778576-62-8
- AN-2728
Catalog No.:BCC1361
CAS No.:906673-24-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 139755-83-2 | SDF | Download SDF |
| PubChem ID | 5212 | Appearance | Powder |
| Formula | C22H30N6O4S | M.Wt | 474.58 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | UK-92480 | ||
| Solubility | DMSO : ≥ 29 mg/mL (61.11 mM) H2O : < 0.1 mg/mL (insoluble) *"≥" means soluble, but saturation unknown. | ||
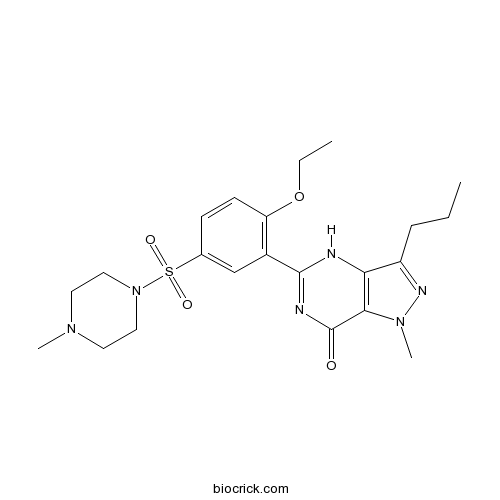
| Chemical Name | 5-[2-ethoxy-5-(4-methylpiperazin-1-yl)sulfonylphenyl]-1-methyl-3-propyl-4H-pyrazolo[4,3-d]pyrimidin-7-one | ||
| SMILES | CCCC1=NN(C2=C1NC(=NC2=O)C3=C(C=CC(=C3)S(=O)(=O)N4CCN(CC4)C)OCC)C | ||
| Standard InChIKey | BNRNXUUZRGQAQC-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H30N6O4S/c1-5-7-17-19-20(27(4)25-17)22(29)24-21(23-19)16-14-15(8-9-18(16)32-6-2)33(30,31)28-12-10-26(3)11-13-28/h8-9,14H,5-7,10-13H2,1-4H3,(H,23,24,29) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sildenafil is a potent phosphodiesterase type 5 (PDE5) inhibitor with IC50 of 5.22 nM.In Vitro:Pretreatment with 1 μM Sildenafil potentiates the phosphorylation of ERK1/ERK2, an increase in the percentage of cells in S phase and cell proliferation, compared with serotonin stimulation alone (P<0.05). Pretreatment with 1 μM Sildenafil citrate followed by serotonin stimulation leads to dramatic increase in OD value to 0.33, significantly different compared with serotonin stimulation alone (P<0.05). 1 μM Sildenafil obviously enhances the upregulation of ERK1/ERK2 phosphorylation induced by serotonin[2].In Vivo:In the dog model of erection, Sildenafil citrate significantly increases ICP and ICP/BP but shows no significant effect on BP compared with vehicle[1]. Sildenafil treatment significantly decreases the number of TL+-cells at 10 but not 0.5 mg/kg. At this time point, cells positive for the M1-like marker COX-2+ are found in the ischemic core in PBS-treated animals, whereas they are mostly observed in the penumbra in 10 mg/kg (but not 0.5 mg/kg) Sildenafil-treated animals. In contrast, 8 days after pMCAo the number of microglia/macrophages stained by Iba-1 are significantly reduced by Sildenafil treatment (0.5 and/or 10 mg/kg dose)[3]. Sildenafil citrate has been reported to decrease flap necrosis in preclinical animal models by increasing the secretion of growth factors (FGF and VEGF), and histologically is shown to be effective in rat cavernous nerve architecture[4]. References: | |||||

Sildenafil Dilution Calculator

Sildenafil Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.1071 mL | 10.5356 mL | 21.0713 mL | 42.1425 mL | 52.6782 mL |
| 5 mM | 0.4214 mL | 2.1071 mL | 4.2143 mL | 8.4285 mL | 10.5356 mL |
| 10 mM | 0.2107 mL | 1.0536 mL | 2.1071 mL | 4.2143 mL | 5.2678 mL |
| 50 mM | 0.0421 mL | 0.2107 mL | 0.4214 mL | 0.8429 mL | 1.0536 mL |
| 100 mM | 0.0211 mL | 0.1054 mL | 0.2107 mL | 0.4214 mL | 0.5268 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Sildenafil is a selective inhibitor of phosphodiesterase-5 (PDE5) [1].
Phosphodiesterases (PDEs) are enzymes that hydrolyzing cyclic nucleotides cAMP and cGMP to their inactive 5’-monofosfatos forms. The PDE5 enzyme is responsible for the hydrolysis of the cGMP, which restores GMP levels [1].
In human umbilical vein endothelial cells (HUVECs), Sildenafil (1 mM/5 h) significantly enhanced NO production, which is ~1.5-fold higher than the effect achieved with insulin (100 nM/1 h) [2].
In male C57BL/6 mice, Sildenafil increased glandular activity of the prostate. The secretory epithelium lining showed tall columnar cells. Moreover, the glandular apical region showed evident secretion vesicles. Also, Sildenafil increased substantially testosterone serum levels [1]. In human corpora cavernosa, Sildenafil inhibited PDE5 with a mean IC50 of 3.9 nM. In 12 patients with erectile dysfunction, Sildenafil enhanced the erectile response, which suggested that PDE5 played an important role in human penile erection. So Sildenafil plays as a new effective treatment for penile erectile dysfunction [3].
References:
[1]. Gomes FO, Carvalho Mda C, Saraiva KL, et al. Effect of chronic Sildenafil treatment on the prostate of C57Bl/6 mice. Tissue Cell, 2014, 46(6): 439-449.
[2]. Mammi C1, Pastore D, Lombardo MF, et al. Sildenafil reduces insulin-resistance in human endothelial cells. PLoS One, 2011, 6(1): e14542.
[3]. Boolell M, Allen MJ, Ballard SA, et al. Sildenafil: an orally active type 5 cyclic GMP-specific phosphodiesterase inhibitor for the treatment of penile erectile dysfunction. Int J Impot Res, 1996, 8(2): 47-52.
- tenuifoliside C
Catalog No.:BCN8299
CAS No.:139726-37-7
- Tenuifoliside B
Catalog No.:BCC9251
CAS No.:139726-36-6
- Tenuifoliside A
Catalog No.:BCN2893
CAS No.:139726-35-5
- Isodunnianol
Catalog No.:BCN6213
CAS No.:139726-30-0
- Dunnianol
Catalog No.:BCN6212
CAS No.:139726-29-7
- BS-181 HCl
Catalog No.:BCC2537
CAS No.:1397219-81-6
- Yunnanxane
Catalog No.:BCN6702
CAS No.:139713-81-8
- Amphotericin B
Catalog No.:BCN2564
CAS No.:1397-89-3
- Gardenine
Catalog No.:BCN6211
CAS No.:139682-36-3
- EPZ005687
Catalog No.:BCC2219
CAS No.:1396772-26-1
- CGP 52432
Catalog No.:BCC6989
CAS No.:139667-74-6
- PR 39 (porcine)
Catalog No.:BCC5856
CAS No.:139637-11-9
- Cucurbitacin E-2-O-Glucoside
Catalog No.:BCC8156
CAS No.:1398-78-3
- Milameline hydrochloride
Catalog No.:BCC7427
CAS No.:139886-04-7
- 3,6'-Disinapoyl sucrose
Catalog No.:BCN2719
CAS No.:139891-98-8
- Globularin
Catalog No.:BCN6215
CAS No.:1399-49-1
- Drahebenine
Catalog No.:BCN7044
CAS No.:1399049-43-4
- Alpinone 3-acetate
Catalog No.:BCN7768
CAS No.:139906-49-3
- Palbinone
Catalog No.:BCN3930
CAS No.:139954-00-0
- Cinnamic acid
Catalog No.:BCN6217
CAS No.:140-10-3
- Nithiamide
Catalog No.:BCC4687
CAS No.:140-40-9
- Pentamidine isethionate
Catalog No.:BCC5644
CAS No.:140-64-7
- 4-Allylanisole
Catalog No.:BCC8674
CAS No.:140-67-0
- Dimethylolurea
Catalog No.:BCC8943
CAS No.:140-95-4
Utero-placental perfusion Doppler indices in growth restricted fetuses: effect of sildenafil citrate.[Pubmed:28345432]
J Matern Fetal Neonatal Med. 2018 Apr;31(8):1045-1050.
OBJECTIVE: To assess efficacy and tolerability of Sildenafil citrate on utero-placental blood flow and fetal growth in pregnancies complicated by fetal growth restriction (FGR). METHODS: From March 2015, a randomized controlled trial of 54 patients at 24 weeks or more complicated by FGR and abnormal Doppler indices were randomly allocated 1:1 into an intervention arm (receive Sildenafil citrate, 50 mg) or a control arm (receive placebo). The primary outcomes were changes occurred in the Doppler parameters 2 h following drug administration. RESULTS: Baseline characteristics were similar between groups. Significant difference was observed in the Delta uterine and umbilical Doppler indices among Sildenafil group as compared to placebo group (p < 0.001). Middle cerebral Doppler indices, however, decreased significantly after Sildenafil, which could be the result of shifting more blood to improve the utero-placental perfusion. No difference regarding Delta cerebro-placental ratio among both groups (p = 0.979). Sildenafil was also associated with pregnancy prolongation (p = .0001), increased gestational age at delivery (p = .004), improved neonatal weight (p = .0001), and less admission to neonatal intensive care unit (p = .03). No adverse effects reported in both treatment arms. CONCLUSION: Sildenafil citrate, by its vasodilator effect, can improve utero-placental blood flow in pregnancies complicated by FGR and abnormal Doppler. CLINICAL TRIAL: gov Registry: NCT02362399.
Sildenafil Enhances Quantity of Immature Neurons and Promotes Functional Recovery in the Developing Ischemic Mouse Brain.[Pubmed:28343223]
Dev Neurosci. 2017;39(1-4):287-297.
BACKGROUND: Hypoxic-ischemic (HI) injury to the developing brain occurs in 1 out of 1,000 live births and remains a major cause of significant morbidity and mortality. A large number of survivors suffer from long-term sequelae including seizures and neurological deficits. However, the pathophysiological mechanisms of recovery after HI insult are not clearly understood, and preventive measures or clinical treatments are nonexistent or not sufficiently effective in the clinical setting. Sildenafil as a specific phosphodiesterase 5 inhibitor leads to increased levels of the second messenger cyclic guanosine monophosphate (cGMP) and promotes functional recovery and neurogenesis after ischemic injury to the adult brain. OBJECTIVE: Here, we investigated the effect of Sildenafil treatment on activation of intracellular signaling pathways, histological and neurogenic response including functional recovery after an ischemic insult to the developing brain. DESIGN/METHODS: Nine-day-old C57BL/6 mice were subjected either to sham operation or underwent ligation of the right common carotid artery followed by hypoxia (8%) for 60 min. Animals were either administered Sildenafil (10 mg/kg, i.p.) or vehicle 2 h after hypoxia. A subgroup of animals received multiple injections of 10 mg/kg daily on 5 consecutive days. Pups were either perfusion fixed at postnatal days 14 or 47 for immunohistochemical analysis, or brains were dissected 2, 6, 12, and 24 h after the end of hypoxia and analyzed for cGMP, pAkt, pGSK-3beta, and beta-catenin by means of ELISA or immunoblotting. In addition, behavioral studies using the wire hang test and elevated plus maze were conducted 21 and 38 days after HI injury. RESULTS: Based on cresyl violet staining, single or multiple Sildenafil injections did not reveal any differences in injury scoring compared to sham animals. However, cerebral levels of cGMP were altered after Sildenafil therapy. Treatment significantly increased numbers of immature neurons, as indicated by doublecortin immunoreactivity in the ipsilateral subventricular zone and striatum. In addition, animals treated with Sildenafil after HI insult demonstrated improved functional recovery. pAkt, pGSK-3beta, and beta-catenin levels vary after HI injury but additional Sildenafil treatment had no impact on protein expression compared to the level of sham controls. CONCLUSIONS: Here, we report that treatment with Sildenafil after HI insult did not improve histological brain injury scores. Nevertheless, our results suggest involvement of the cGMP and PI3K/Akt/GSK-3beta signaling pathway with promotion of a neurogenic response and reduction of neurological deficits. In summary, Sildenafil may have a role in promoting recovery from HI injury in the developing brain.
Treatment response to sildenafil in men with erectile dysfunction relative to concomitant comorbidities and age.[Pubmed:28371025]
Int J Clin Pract. 2017 Mar;71(3-4).
AIM: To evaluate treatment response in men with erectile dysfunction (ED) and concomitant comorbidities. METHODS: Data were pooled from 42 placebo-controlled, flexible-dose Sildenafil trials. In most trials, the Sildenafil dose was 50 mg, taken ~1 hour before sexual activity but not more than once daily, with adjustment to 100 or 25 mg as needed. The overall population (N=9413) was stratified by age (<45, 46-64, >/=65 years). Treatment response was defined as a minimal clinically important difference (MCID) from baseline in the International Index of Erectile Function-Erectile Function (IIEF-EF) domain score of >2, >5 and >7 for men with mild, moderate and severe ED at baseline, respectively, or an IIEF-EF domain score >/=26 (no ED) at end-point. RESULTS: In the overall population, treatment response using the IIEF-EF MCID definition was significantly greater (P<.0001) with Sildenafil vs placebo in men with no comorbidity (77% vs 33%), cardiovascular disease/hypertension only (71% vs 27%), diabetes only (63% vs 24%) or depression only (78% vs 29%). Using an IIEF-EF score >/=26, treatment response was significantly greater (P<.0001) with Sildenafil vs placebo in men with no comorbidity (49% vs 17%), cardiovascular disease/hypertension only (48% vs 12%), diabetes only (40% vs 12%) or depression only (60% vs 17%). With each definition, the treatment response for each age and comorbidity was significantly greater (PSildenafil vs placebo. CONCLUSION: The treatment response was significantly greater with Sildenafil vs placebo in men with ED and each comorbidity regardless of age.


