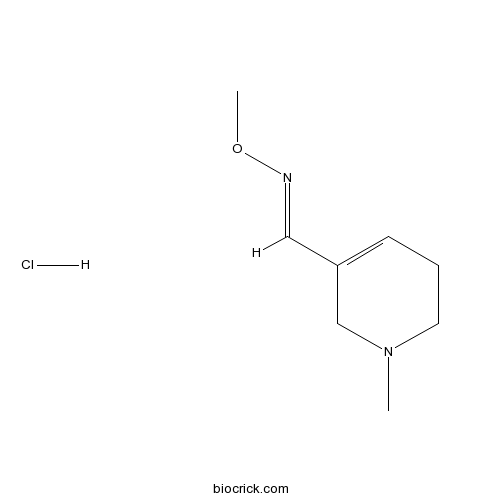
Milameline hydrochlorideMuscarinic receptor agonist CAS# 139886-04-7 |
- Meprednisone
Catalog No.:BCC4893
CAS No.:1247-42-3
- Hydrocortisone
Catalog No.:BCN2192
CAS No.:50-23-7
- Prednisolone
Catalog No.:BCC4830
CAS No.:50-24-8
- Desonide
Catalog No.:BCC4967
CAS No.:638-94-8
- Fluticasone propionate
Catalog No.:BCC4907
CAS No.:80474-14-2
- Methylprednisolone
Catalog No.:BCC2256
CAS No.:83-43-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 139886-04-7 | SDF | Download SDF |
| PubChem ID | 9571001 | Appearance | Powder |
| Formula | C8H15ClN2O | M.Wt | 190.67 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in water | ||
| Chemical Name | (E)-N-methoxy-1-(1-methyl-3,6-dihydro-2H-pyridin-5-yl)methanimine;hydrochloride | ||
| SMILES | CN1CCC=C(C1)C=NOC.Cl | ||
| Standard InChIKey | WEBMRZODPLSRKR-MLBSPLJJSA-N | ||
| Standard InChI | InChI=1S/C8H14N2O.ClH/c1-10-5-3-4-8(7-10)6-9-11-2;/h4,6H,3,5,7H2,1-2H3;1H/b9-6+; | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Muscarinic receptor agonist that displays roughly equal affinity at all receptor subtypes (Ki values are 2.3, 2.4, 3.6, 3.8 and 4.3 μM for hM1, hM2, hM3, hM4 and hM5 receptors respectively). Cognitive enhancer; reverses spatial memory deficits in rats. |

Milameline hydrochloride Dilution Calculator

Milameline hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 5.2447 mL | 26.2233 mL | 52.4466 mL | 104.8933 mL | 131.1166 mL |
| 5 mM | 1.0489 mL | 5.2447 mL | 10.4893 mL | 20.9787 mL | 26.2233 mL |
| 10 mM | 0.5245 mL | 2.6223 mL | 5.2447 mL | 10.4893 mL | 13.1117 mL |
| 50 mM | 0.1049 mL | 0.5245 mL | 1.0489 mL | 2.0979 mL | 2.6223 mL |
| 100 mM | 0.0524 mL | 0.2622 mL | 0.5245 mL | 1.0489 mL | 1.3112 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Cucurbitacin E-2-O-Glucoside
Catalog No.:BCC8156
CAS No.:1398-78-3
- Sildenafil
Catalog No.:BCC1947
CAS No.:139755-83-2
- tenuifoliside C
Catalog No.:BCN8299
CAS No.:139726-37-7
- Tenuifoliside B
Catalog No.:BCC9251
CAS No.:139726-36-6
- Tenuifoliside A
Catalog No.:BCN2893
CAS No.:139726-35-5
- Isodunnianol
Catalog No.:BCN6213
CAS No.:139726-30-0
- Dunnianol
Catalog No.:BCN6212
CAS No.:139726-29-7
- BS-181 HCl
Catalog No.:BCC2537
CAS No.:1397219-81-6
- Yunnanxane
Catalog No.:BCN6702
CAS No.:139713-81-8
- Amphotericin B
Catalog No.:BCN2564
CAS No.:1397-89-3
- Gardenine
Catalog No.:BCN6211
CAS No.:139682-36-3
- EPZ005687
Catalog No.:BCC2219
CAS No.:1396772-26-1
- 3,6'-Disinapoyl sucrose
Catalog No.:BCN2719
CAS No.:139891-98-8
- Globularin
Catalog No.:BCN6215
CAS No.:1399-49-1
- Drahebenine
Catalog No.:BCN7044
CAS No.:1399049-43-4
- Alpinone 3-acetate
Catalog No.:BCN7768
CAS No.:139906-49-3
- Palbinone
Catalog No.:BCN3930
CAS No.:139954-00-0
- Cinnamic acid
Catalog No.:BCN6217
CAS No.:140-10-3
- Nithiamide
Catalog No.:BCC4687
CAS No.:140-40-9
- Pentamidine isethionate
Catalog No.:BCC5644
CAS No.:140-64-7
- 4-Allylanisole
Catalog No.:BCC8674
CAS No.:140-67-0
- Dimethylolurea
Catalog No.:BCC8943
CAS No.:140-95-4
- Nystatin (Fungicidin)
Catalog No.:BCC4813
CAS No.:1400-61-9
- 4μ8C
Catalog No.:BCC4754
CAS No.:14003-96-4
Milameline (CI-979/RU35926): a muscarinic receptor agonist with cognition-activating properties: biochemical and in vivo characterization.[Pubmed:10525104]
J Pharmacol Exp Ther. 1999 Nov;291(2):812-22.
Milameline (E-1,2,5,6-tetrahydro-1-methyl-3-pyridinecarboxaldehyde, O-methyloxime monohydrochloride, CI-979, PD129409, RU35926) was characterized in vitro and evaluated for effects on central and peripheral cholinergic activity in rats and rhesus monkeys. In muscarinic binding studies, milameline displayed nanomolar affinity with an agonist ligand and micromolar affinity with antagonist ligands, with approximately equal affinities determined at the five subtypes of human muscarinic receptors (hM(1)-hM(5)) with whole cells or membranes from stably transfected Chinese hamster ovary (CHO) cells. On binding, milameline stimulated phosphatidylinositol hydrolysis in hM(1) and hM(3) CHO cells and inhibited forskolin-activated cAMP accumulation in hM(2) and hM(4) CHO cells. Additionally, it decreased K(+)-stimulated release of [(3)H]acetylcholine from rat cortical slices. Responses were not caused by the inhibition of acetylcholinesterase, and there was no significant binding to approximately 30 other neurotransmitter binding sites. In rats, milameline decreased spontaneous and scopolamine-induced swimming activity, improved water-maze performance of animals impaired by basal forebrain lesions, increased cortical blood flow, decreased core body temperature, and increased gastrointestinal motility. Electroencephalogram activity in both rats and monkeys was characterized by a predominance of low-voltage desynchronized activity consistent with an increase in arousal. Milameline also reversed a scopolamine-induced impairment of attention on a continuous-performance task in monkeys. Thus, milameline possesses a pharmacological profile consistent with that of a partial muscarinic agonist, with central cholinergic actions being produced in rats and monkeys at doses slightly lower than those stimulating peripheral cholinergic receptors.
Functional comparison of muscarinic partial agonists at muscarinic receptor subtypes hM1, hM2, hM3, hM4 and hM5 using microphysiometry.[Pubmed:10323594]
Br J Pharmacol. 1999 Apr;126(7):1620-4.
1. This study describes the pharmacological comparison of the muscarinic partial agonists sabcomeline, xanomeline and milameline at human cloned muscarinic receptor subtypes (hM1-5). 2. Radioligand binding studies at the hM1-5 muscarinic receptor subtypes were compared with functional studies using microphysiometry using carbachol as the standard full agonist. 3. In binding assays none of the compounds studied displayed preferential affinity for the M1,3,4 or M5 subtypes although carbachol was less potent at hM1 than hM3,4,5. 4. In functional studies, all of the compounds studied displayed similar levels of efficacy across the muscarinic receptors with the exception of M3, where there was a large apparent receptor reserve and the compounds behaved essentially as full agonists. 5. Sabcomeline was the most potent agonist in functional studies but also showed the lowest efficacy. In terms of potency, xanomeline showed some selectivity for M1 over M2 receptors and milameline showed some selectivity for M2 over M1 receptors. 6. These results show the value of microphysiometry in being able to compare receptor pharmacology across subtypes irrespective of the signal transduction pathway. 7. None of the partial agonists showed functional selectivity for M1 receptors, or indeed any muscarinic receptor, in the present study.
Preclinical and phase 1 clinical characterization of CI-979/RU35926, a novel muscarinic agonist for the treatment of Alzheimer's disease.[Pubmed:10188788]
Life Sci. 1995;56(11-12):877-82.
In vitro and in vivo characterization in rodents and monkeys shows that CI-979/RU35926 is a partial muscarinic agonist with equal affinity for the five subtypes of muscarinic receptors. It activates central cholinergic receptors as shown by its ability to decrease body temperature, enhance local cortical blood flow and increase cortical arousal measured by QEEG. Further, it reverses spatial memory deficits in rats with ibotenic acid-induced lesions of forebrain cholinergic neurons. Signs of peripheral cholinergic stimulation appear at doses higher or equal to those necessary to produce central activity. In a single-dose tolerance study in young, healthy human volunteers, CI-979/RU35926 was well tolerated at doses of 0.002-1.0 mg with cholinergic symptoms such as hypersalivation and sweating, observed at 2-4 mg. It demonstrated linear pharmacokinetic behavior over a dose range of 0.1 to 4 mg and elimination half-life varied from 2-5 hours. Measurement of unchanged drug in urine suggests that the drug was extensively metabolized. Thus, the safety profile supported further clinical evaluation and CI-979/RU35926 is currently in Phase II clinical trials.


