EurycomalactoneCAS# 23062-24-0 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 23062-24-0 | SDF | Download SDF |
| PubChem ID | 441793 | Appearance | Cryst. |
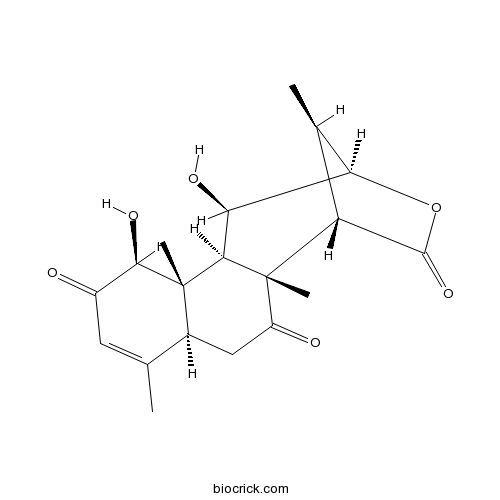
| Formula | C19H24O6 | M.Wt | 348.4 |
| Type of Compound | Diterpenoids | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| SMILES | CC1C2C(C3C4(C(CC(=O)C3(C1C(=O)O2)C)C(=CC(=O)C4O)C)C)O | ||
| Standard InChIKey | OGHYZHNTIINXEO-MGBQOKOWSA-N | ||
| Standard InChI | InChI=1S/C19H24O6/c1-7-5-10(20)16(23)18(3)9(7)6-11(21)19(4)12-8(2)14(25-17(12)24)13(22)15(18)19/h5,8-9,12-16,22-23H,6H2,1-4H3/t8-,9+,12-,13+,14-,15-,16-,18+,19+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | 1. Eurycomalactone displays potent activity against all the tested cell lines [colon 26-L5 carcinoma (IC50 = 0.70 microM), B16-BL6 melanoma (IC50 = 0.59 microM), Lewis lung carcinoma (IC50 = 0.78 microM), and a human lung A549 adenocarcinoma (IC50 = 0.73 microM)]. 2. Eurycomalactone as a potent NF-κB inhibitor with the IC50 value of less than 1 uM. 3. Eurycomalactone displays antiplasmodial activity against Gombak A isolate. |
| Targets | NF-kB | Antifection |

Eurycomalactone Dilution Calculator

Eurycomalactone Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.8703 mL | 14.3513 mL | 28.7026 mL | 57.4053 mL | 71.7566 mL |
| 5 mM | 0.5741 mL | 2.8703 mL | 5.7405 mL | 11.4811 mL | 14.3513 mL |
| 10 mM | 0.287 mL | 1.4351 mL | 2.8703 mL | 5.7405 mL | 7.1757 mL |
| 50 mM | 0.0574 mL | 0.287 mL | 0.5741 mL | 1.1481 mL | 1.4351 mL |
| 100 mM | 0.0287 mL | 0.1435 mL | 0.287 mL | 0.5741 mL | 0.7176 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Varenicline Hydrochloride
Catalog No.:BCC4156
CAS No.:230615-23-3
- Sinensetin
Catalog No.:BCN6356
CAS No.:2306-27-6
- (-)-Sophoranone
Catalog No.:BCN7162
CAS No.:23057-55-8
- L-AP4
Catalog No.:BCC6550
CAS No.:23052-81-5
- Dihydroconiferyl alcohol
Catalog No.:BCN7047
CAS No.:2305-13-7
- Lofepramine
Catalog No.:BCC7402
CAS No.:23047-25-8
- Ajugasterone C
Catalog No.:BCN2757
CAS No.:23044-80-6
- Z-Arg(NO2)-OH
Catalog No.:BCC3063
CAS No.:2304-98-5
- Z-Asn-OH
Catalog No.:BCC2794
CAS No.:2304-96-3
- Z-β-Ala-OH
Catalog No.:BCC3058
CAS No.:2304-94-1
- Terbutaline Sulfate
Catalog No.:BCC4320
CAS No.:23031-32-5
- Apelin-36 (rat, mouse)
Catalog No.:BCC5911
CAS No.:230299-95-3
- PD 102807
Catalog No.:BCC7145
CAS No.:23062-91-1
- 4-Amino-N-methylphthalimide
Catalog No.:BCC8686
CAS No.:2307-00-8
- Xylazine HCl
Catalog No.:BCC4341
CAS No.:23076-35-9
- Sitosteryl palmitate
Catalog No.:BCN5078
CAS No.:2308-85-2
- 2-amino-3-(3-bromo-5-chloro-4-hydroxyphenyl)propanoic acid
Catalog No.:BCN8284
CAS No.:
- Corilagin
Catalog No.:BCN2322
CAS No.:23094-69-1
- Chebulagic acid
Catalog No.:BCN3262
CAS No.:23094-71-5
- Neuropeptide SF (mouse, rat)
Catalog No.:BCC6054
CAS No.:230960-31-3
- UK 356618
Catalog No.:BCC2378
CAS No.:230961-08-7
- UK 370106
Catalog No.:BCC2379
CAS No.:230961-21-4
- Fumagillin
Catalog No.:BCC2347
CAS No.:23110-15-8
- Methylxanthoxylin
Catalog No.:BCC8212
CAS No.:23121-32-6
Antiplasmodial studies of Eurycoma longifolia Jack using the lactate dehydrogenase assay of Plasmodium falciparum.[Pubmed:15138004]
J Ethnopharmacol. 2004 Jun;92(2-3):223-7.
The roots of Eurycoma longifolia Jack have been used as traditional medicine to treat malaria. A systematic bioactivity-guided fractionation of this plant was conducted involving the determination of the effect of its various extracts and their chemical constituents on the lactate dehydrogenase activity of in vitro chloroquine-resistant Gombak A isolate and chloroquine-sensitive D10 strain of Plasmodium falciparum parasites. Their antiplasmodial activity was also compared with their known in vitro cytotoxicity against KB cells. Four quassinoids, eurycomanone (1), 13,21-dihydroeurycomanone (3), 13 alpha(21)-epoxyeurycomanone (4), Eurycomalactone (6) and an alkaloid, 9-methoxycanthin-6-one (7), displayed higher antiplasmodial activity against Gombak A isolate but were less active against the D10 strain when compared with chloroquine. Amongst the compounds tested, 1 and 3 showed higher selectivity indices obtained for the cytotoxicity to antiplasmodial activity ratio than 14,15 beta-dihydroxyklaineanone (2), eurycomanol (5), 6 and 7.
Cytotoxic activity of quassinoids from Eurycoma longifolia.[Pubmed:20734929]
Nat Prod Commun. 2010 Jul;5(7):1009-12.
Twenty-four quassinoids isolated from Eurycoma longifolia Jack were investigated for their cytotoxicity against a panel of four different cancer cell lines, which includes three murine cell lines [colon 26-L5 carcinoma (colon 26-L5), B16-BL6 melanoma (B16-BL6), Lewis lung carcinoma (LLC)] and a human lung A549 adenocarcinoma (A549) cell line. Among the tested compounds, Eurycomalactone (9) displayed the most potent activity against all the tested cell lines; colon 26-L5 (IC50 = 0.70 microM), B16-BL6 (IC50 = 0.59 microM), LLC (IC50 = 0.78 microM), and A549 (IC50 = 0.73 microM). These activities were comparable to clinically used anticancer agent doxorubicin (colon 26-L5, IC50 = 0.76 microM; B16-BL6, IC50 = 0.86 microM; LLC, IC50 = 0.80 microM; A549, IC50 = 0.66 microM).
Tongkat Ali (Eurycoma longifolia Jack): a review on its ethnobotany and pharmacological importance.[Pubmed:20434529]
Fitoterapia. 2010 Oct;81(7):669-79.
Eurycoma longifolia Jack is an herbal medicinal plant of South-East Asian origin, popularly recognized as 'Tongkat Ali.' The plant parts have been traditionally used for its antimalarial, aphrodisiac, anti-diabetic, antimicrobial and anti-pyretic activities, which have also been proved scientifically. The plant parts are rich in various bioactive compounds (like eurycomaoside, eurycolactone, Eurycomalactone, eurycomanone, and pasakbumin-B) among which the alkaloids and quassinoids form a major portion. Even though toxicity and safety evaluation studies have been pursued, still a major gap exists in providing scientific base for commercial utilization and clearance of the Tongkat Ali products with regard to consumer's safety. The present review aims at reviewing the research works undertaken till date, on this plant in order to provide sufficient baseline information for future works and for commercial exploitation.
NF-kappaB inhibitors from Eurycoma longifolia.[Pubmed:24467387]
J Nat Prod. 2014 Mar 28;77(3):483-8.
The roots of Eurycoma longifolia have been used in many countries of Southeast Asia to alleviate various diseases including malaria, dysentery, sexual insufficiency, and rheumatism. Although numerous studies have reported the pharmacological properties of E. longifolia, the mode of action of the anti-inflammatory activity has not been elucidated. Bioguided isolation of NF-kappaB inhibitors using an NF-kappaB-driven luciferase reporter gene assay led to the identification of a new quassinoid, eurycomalide C (1), together with 27 known compounds including 11 quassinoids (2-12), six alkaloids (13-18), two coumarins (19, 20), a squalene derivative (21), a triterpenoid (22), and six phenolic compounds (23-28) from the extract of E. longifolia. Evaluation of the biological activity revealed that C19-type and C20-type quassinoids, beta-carboline, and canthin-6-one alkaloids are potent NF-kappaB inhibitors, with IC50 values in the low micromolar range, while C18-type quassinoids, phenolic compounds, coumarins, the squalene derivative, and the triterpenoid turned out to be inactive when tested at a concentration of 30 muM. Eurycomalactone (2), 14,15beta-dihydroklaieanone (7), and 13,21-dehydroeurycomanone (10) were identified as potent NF-kappaB inhibitors with IC50 values of less than 1 muM.
Simultaneous quantitation of six major quassinoids in Tongkat Ali dietary supplements by liquid chromatography with tandem mass spectrometry.[Pubmed:25914245]
J Sep Sci. 2015 Jul;38(13):2260-6.
Tongkat Ali (Eurycoma longifolia) is one of the most popular traditional herbs in Southeast Asia and generally consumed as forms of dietary supplements, tea, or drink additives for coffee or energy beverages. In this study, the liquid chromatography with tandem mass spectrometry method for the simultaneous quantitation of six major quassinoids of Tongkat Ali (eurycomanone, 13,21-dihydroeurycomanone, 13alpha(21)-epoxyeurycomanone, 14,15beta-dihydroxyklaineanone, Eurycomalactone, and longilactone) was developed and validated. Using the developed method, the content of the six quassinoids was measured in Tongkat Ali containing dietary supplement tablets or capsules, and the resulting data were used to confirm the presence of Tongkat Ali in those products. Among the six quassinoids, eurycomanone was the most abundant quassinoid in all samples tested. The developed method would be useful for the quality assessment of Tongkat Ali containing dietary supplements.


