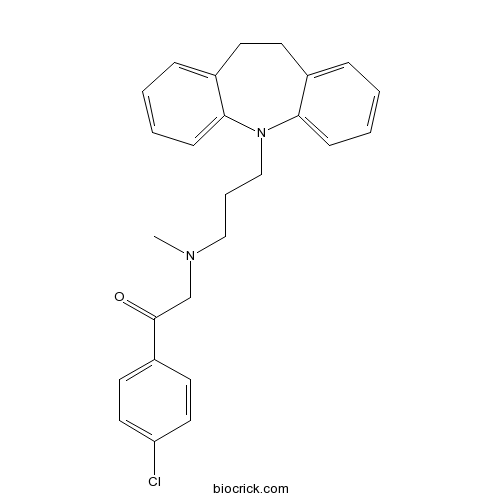
Lofepramine5-HT and noradrenalin re-uptake inhibitor (SNRI) CAS# 23047-25-8 |
- Apicidin
Catalog No.:BCC3599
CAS No.:183506-66-3
- Scriptaid
Catalog No.:BCC2163
CAS No.:287383-59-9
- Mocetinostat (MGCD0103, MG0103)
Catalog No.:BCC2146
CAS No.:726169-73-9
- PCI-24781 (CRA-024781)
Catalog No.:BCC2155
CAS No.:783355-60-2
- JNJ-26481585
Catalog No.:BCC2147
CAS No.:875320-29-9
- Droxinostat
Catalog No.:BCC2157
CAS No.:99873-43-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 23047-25-8 | SDF | Download SDF |
| PubChem ID | 3947 | Appearance | Powder |
| Formula | C26H27N2OCl | M.Wt | 418.97 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Lopramine, Leo 640 | ||
| Solubility | Soluble to 75 mM in DMSO and to 10 mM in ethanol | ||
| Chemical Name | 1-(4-chlorophenyl)-2-[3-(5,6-dihydrobenzo[b][1]benzazepin-11-yl)propyl-methylamino]ethanone | ||
| SMILES | CN(CCCN1C2=CC=CC=C2CCC3=CC=CC=C31)CC(=O)C4=CC=C(C=C4)Cl | ||
| Standard InChIKey | SAPNXPWPAUFAJU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C26H27ClN2O/c1-28(19-26(30)22-13-15-23(27)16-14-22)17-6-18-29-24-9-4-2-7-20(24)11-12-21-8-3-5-10-25(21)29/h2-5,7-10,13-16H,6,11-12,17-19H2,1H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Serotonin and noradrenalin re-uptake inhibitor (SNRI) that is metabolized to desipramine. Produces inhibition of liver tryptophan pyrollase activity in vitro and displays antidepressant properties in vivo. |

Lofepramine Dilution Calculator

Lofepramine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3868 mL | 11.934 mL | 23.8681 mL | 47.7361 mL | 59.6701 mL |
| 5 mM | 0.4774 mL | 2.3868 mL | 4.7736 mL | 9.5472 mL | 11.934 mL |
| 10 mM | 0.2387 mL | 1.1934 mL | 2.3868 mL | 4.7736 mL | 5.967 mL |
| 50 mM | 0.0477 mL | 0.2387 mL | 0.4774 mL | 0.9547 mL | 1.1934 mL |
| 100 mM | 0.0239 mL | 0.1193 mL | 0.2387 mL | 0.4774 mL | 0.5967 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Ajugasterone C
Catalog No.:BCN2757
CAS No.:23044-80-6
- Z-Arg(NO2)-OH
Catalog No.:BCC3063
CAS No.:2304-98-5
- Z-Asn-OH
Catalog No.:BCC2794
CAS No.:2304-96-3
- Z-β-Ala-OH
Catalog No.:BCC3058
CAS No.:2304-94-1
- Terbutaline Sulfate
Catalog No.:BCC4320
CAS No.:23031-32-5
- Apelin-36 (rat, mouse)
Catalog No.:BCC5911
CAS No.:230299-95-3
- Physalin A
Catalog No.:BCN7920
CAS No.:23027-91-0
- Neoglycyrol
Catalog No.:BCN2907
CAS No.:23013-84-5
- MPTP hydrochloride
Catalog No.:BCC1778
CAS No.:23007-85-4
- Atazanavir sulfate (BMS-232632-05)
Catalog No.:BCC2114
CAS No.:229975-97-7
- AGN 194310
Catalog No.:BCC5416
CAS No.:229961-45-9
- GW9662
Catalog No.:BCC2260
CAS No.:22978-25-2
- Dihydroconiferyl alcohol
Catalog No.:BCN7047
CAS No.:2305-13-7
- L-AP4
Catalog No.:BCC6550
CAS No.:23052-81-5
- (-)-Sophoranone
Catalog No.:BCN7162
CAS No.:23057-55-8
- Sinensetin
Catalog No.:BCN6356
CAS No.:2306-27-6
- Varenicline Hydrochloride
Catalog No.:BCC4156
CAS No.:230615-23-3
- Eurycomalactone
Catalog No.:BCN3108
CAS No.:23062-24-0
- PD 102807
Catalog No.:BCC7145
CAS No.:23062-91-1
- 4-Amino-N-methylphthalimide
Catalog No.:BCC8686
CAS No.:2307-00-8
- Xylazine HCl
Catalog No.:BCC4341
CAS No.:23076-35-9
- Sitosteryl palmitate
Catalog No.:BCN5078
CAS No.:2308-85-2
- 2-amino-3-(3-bromo-5-chloro-4-hydroxyphenyl)propanoic acid
Catalog No.:BCN8284
CAS No.:
- Corilagin
Catalog No.:BCN2322
CAS No.:23094-69-1
Cost-effectiveness and cost-utility of tricyclic antidepressants, selective serotonin reuptake inhibitors and lofepramine: randomised controlled trial.[Pubmed:16582060]
Br J Psychiatry. 2006 Apr;188:337-45.
BACKGROUND: The cost-effectiveness of tricyclic antidepressants (TCAs) and selective serotonin reuptake inhibitors (SSRIs) has not been compared in a prospective study in primary care. AIMS: To determine the relative cost-effectiveness of TCAs, SSRIs and Lofepramine in UK primary care. METHOD: An open-label, three-arm randomised trial with a preference arm. Practitioners referred 327 patients with incident depression. RESULTS: No significant differences were found in effectiveness or cost-effectiveness. The numbers of depression-free weeks over 12 months (on the Hospital Anxiety and Depression Scale) were 25.3 (95% CI 21.3-29.0) for TCAs, 28.3 (95% CI 24.3-32.2) for SSRIs and 24.6 (95% CI 20.6-28.9) for Lofepramine. Mean health service costs per patient were pound 762 (95% CI 553-1059) for TCAs, pound 875 (95% CI 675-1355) for SSRIs and pound 867 (95% CI 634-1521) for Lofepramine. Cost-effectiveness acceptability curves suggested SSRIs were most cost-effective (with a probability of up to 0.6). CONCLUSIONS: The findings support a policy of recommending SSRIs as first-choice antidepressants in primary care.
Treatment of multiple sclerosis with lofepramine, L-phenylalanine and vitamin B(12): mechanism of action and clinical importance: roles of the locus coeruleus and central noradrenergic systems.[Pubmed:12376086]
Med Hypotheses. 2002 Nov;59(5):594-602.
In a randomized, placebo-controlled double-blind trial a combination of Lofepramine, phenylalanine and vitamin B(12) was found to be effective in relieving the symptoms of multiple sclerosis (MS). The effect occurred within 2-4 weeks, and improved all types of symptoms in all types of MS. The combination was also effective in relieving symptoms in patients with chronic pain and chronic fatigue. We hypothesize that the action of this combined therapy may relate to activation of the noradrenergic locus coeruleus/lateral tegmentum (LC/LT) system which has the potential to influence the functioning of large areas of the brain and spinal cord.
A randomised controlled trial to compare the cost-effectiveness of tricyclic antidepressants, selective serotonin reuptake inhibitors and lofepramine.[Pubmed:15876362]
Health Technol Assess. 2005 May;9(16):1-134, iii.
OBJECTIVE: To determine the relative cost-effectiveness of three classes of antidepressants: tricyclic antidepressants (TCAs), selective serotonin reuptake inhibitors (SSRIs), and the modified TCA Lofepramine, as first choice treatments for depression in primary care. DESIGN: Open, pragmatic, controlled trial with three randomised arms and one preference arm. Patients were followed up for 12 months. SETTING: UK primary care: 73 practices in urban and rural areas in England. PARTICIPANTS: Patients with a new episode of depressive illness according to GP diagnosis. INTERVENTIONS: Patients were randomised to receive a TCA (amitriptyline, dothiepin or imipramine), an SSRI (fluoxetine, sertraline or paroxetine) or Lofepramine. Patients or GPs were able to choose an alternative treatment if preferred. MAIN OUTCOME MEASURES: At baseline the Clinical Interview Schedule, Revised (CIS-R PROQSY computerised version) was administered to establish symptom profiles. Outcome measures over the 12-month follow-up included the Hospital Anxiety and Depression Scale self-rating of depression (HAD-D), CIS-R, EuroQol (EQ-5D) for quality of life, Short Form (SF-36) for generic health status, and patient and practice records of use of health and social services. The primary effectiveness outcome was the number of depression-free weeks (HAD-D less than 8, with interpolation of intervening values) and the primary cost outcome total direct NHS costs. Quality-adjusted life-years (QALYs) were used as the outcome measure in a secondary analysis. Incremental cost-effectiveness ratios and cost-effectiveness acceptability curves were computed. Estimates were bootstrapped with 5000 replications. RESULTS: In total, 327 patients were randomised. Follow-up rates were 68% at 3 months and 52% at 1 year. Linear regression analysis revealed no significant differences between groups in number of depression-free weeks when adjusted for baseline HAD-D. A higher proportion of patients randomised to TCAs entered the preference arm than those allocated to the other choices. Switching to another class of antidepressant in the first few weeks of treatment occurred significantly more often in the Lofepramine arm and less in the preference arm. There were no significant differences between arms in mean cost per depression-free week. For values placed on an additional QALY of over 5000 pounds, treatment with SSRIs was likely to be the most cost-effective strategy. TCAs were the least likely to be cost-effective as first choice of antidepressant for most values of a depression-free week or QALY respectively, but these differences were relatively modest. CONCLUSIONS: When comparing the different treatment options, no significant differences were found in outcomes or costs within the sample, but when outcomes and costs were analysed together, the resulting cost-effectiveness acceptability curves suggested that SSRIs were likely to be the most cost-effective option, although the probability of this did not rise above 0.6. Choosing Lofepramine is likely to lead to a greater proportion of patients switching treatment in the first few weeks. Further research is still needed on the management of depressive illness in primary care. This should address areas such as the optimum severity threshold at which medication should be used; the feasibility and effectiveness of adopting structured depression management programmes in the UK context; the importance of factors such as physical co-morbidity and recent life events in GPs' prescribing decisions; alternative ways of collecting data; and the factors that give rise to many patients being reluctant to accept medication and discontinue treatment early.
A randomised placebo controlled exploratory study of vitamin B-12, lofepramine, and L-phenylalanine (the "Cari Loder regime") in the treatment of multiple sclerosis.[Pubmed:12185153]
J Neurol Neurosurg Psychiatry. 2002 Sep;73(3):246-9.
OBJECTIVE: To determine whether combination therapy with Lofepramine, L-phenylalanine, and intramuscular vitamin B-12 (the "Cari Loder regime") reduces disability in patients with multiple sclerosis. METHODS: A placebo controlled, double blind, randomised study carried out in five United Kingdom centres on outpatients with clinically definite multiple sclerosis, measurable disability on Guy's neurological disability scale (GNDS), no relapse in the preceding six months, and not on antidepressant drugs. Over 24 weeks all patients received vitamin B-12, 1 mg intramuscularly weekly, and either Lofepramine 70 mg and L-phenylalanine 500 mg twice daily, or matching placebo tablets. Outcome was assessed using the GNDS, the Kurtzke expanded disability status scale; the Beck depression inventory, the Chalder fatigue scale, and the Gulick MS specific symptom scale. RESULTS: 138 patients were entered, and two were lost from each group. There was no statistically significant difference between the groups at entry or at follow up. Analysis of covariance suggested that treated patients had better outcomes on four of the five scales used. Both groups showed a reduction of 2 GNDS points within the first two weeks, and when data from all time points were considered, the treated group had a significant improvement of 0.6 GNDS points from two weeks onwards. CONCLUSIONS: Patients with multiple sclerosis improved by 2 GNDS points after starting vitamin B-12 injections. The addition of Lofepramine and L-phenylalanine added a further 0.6 points benefit. More research is needed to confirm and explore the significance of this clinically small difference.
An investigation of the antidepressant properties of lofepramine and its desmethylated metabolites in the forced swim and olfactory bulbectomized rat models of depression.[Pubmed:10082234]
Eur Neuropsychopharmacol. 1999 Jan;9(1-2):101-5.
The purpose of this study was to examine the metabolites of Lofepramine (LOF), namely desipramine (DMI), desmethyl desipramine (DDMI) and desmethyl Lofepramine (DML) in the forced swim and olfactory bulbectomized (OB) rat models of depression. In the first study, subacute treatment with DMI (10 mg/kg) and DML (20 mg/kg), but not LOF, reduced the immobility time in the forced swim test. In the "open field", chronic (14 day) treatment with all drugs attenuated the hyperactivity associated with olfactory bulbectomy. In the second experiment, a lower dose of DML (10 mg/kg) demonstrated activity following subacute treatment in the forced swim and following chronic treatment in the OB model. In addition, DDMI (10 mg/kg) was active in both models. From these results, it can be concluded that, at the doses employed, LOF and it's desmethylated metabolites, DMI, DML and DDMI, exhibits activity in the OB model. In contrast, Lofepramine but not it's desmethylated derivatives is inactive in the forced swim test, perhaps suggesting the requirement of metabolic conversion of LOF to reveal antidepressant activity in this model.
The effects of lofepramine and desmethylimipramine on tryptophan metabolism and disposition in the rat.[Pubmed:1867646]
Biochem Pharmacol. 1991 Jul 25;42(4):921-9.
Acute and chronic administration of Lofepramine and its major metabolite desmethylimipramine (DMI) to rats elevates brain tryptophan concentration, thereby enhancing cerebral 5-hydroxytryptamine (5-HT) synthesis, by increasing the availability of circulating tryptophan to the brain, secondarily to inhibition of liver tryptophan pyrrolase (tryptophan 2,3-dioxygenase, L-tryptophan:O2 oxidoreductase, decyclizing; EC 1.13.11.11) activity. The pyrrolase inhibition by Lofepramine occurs independently of metabolism to DMI, because it can be demonstrated directly in vitro. Lofepramine also differs from DMI in its action profile on the above and related aspects of tryptophan metabolism and disposition. These results demonstrate that Lofepramine influences tryptophan and 5-HT metabolism and disposition independently of its major metabolite DMI, and are discussed briefly in relation to the mechanism of action of antidepressants.
A comparison of the pharmacological properties of the novel tricyclic antidepressant lofepramine with its major metabolite, desipramine: a review.[Pubmed:2891742]
Int Clin Psychopharmacol. 1987 Oct;2(4):281-97.
The effects of the novel tricyclic antidepressant Lofepramine were compared with that of its principal metabolite desipramine. In double-bind clinical trials, Lofepramine has been shown to be as effective as desipramine and other comparator tricyclic antidepressants in the treatment of endogenous and reactive depression, but there are some differences between them. Thus the acute toxicity of Lofepramine is approximately one-fifth that of its metabolite; Lofepramine is a less potent muscarinic receptor antagonist than desipramine (verified by clinical studies in volunteers and depressed patients); Lofepramine is less likely to produce conduction defects than desipramine. Neurochemical studies show that both Lofepramine and its metabolite are potent noradrenaline uptake inhibitors in vitro and evidence is presented to suggest that Lofepramine may release this amine following chronic administration in vivo; both drugs slightly increase serotonin turnover under these conditions and down-regulate cortical beta-adrenoceptor function. Unlike desipramine and most clinically effective antidepressants, Lofepramine was inactive in attenuating the hyperactivity of olfactory bulbectomized rats in the "open field" apparatus, and in reversing acute clonidine induced hypomotility. From such tests it appears unlikely that the active metabolite, desipramine, is formed in the brain in sufficient concentrations after chronic Lofepramine administration to make a substantial contribution towards the pharmacological activity of the parent compound.


