Terbutaline SulfateCAS# 23031-32-5 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 23031-32-5 | SDF | Download SDF |
| PubChem ID | 441334 | Appearance | Powder |
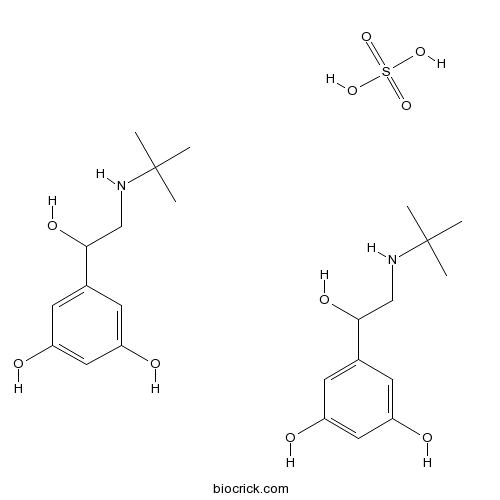
| Formula | C24H40N2O10S | M.Wt | 548.65 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : 100 mg/mL (364.54 mM; Need ultrasonic) DMSO : 5 mg/mL (18.23 mM; Need ultrasonic) | ||
| Chemical Name | 5-[2-(tert-butylamino)-1-hydroxyethyl]benzene-1,3-diol;sulfuric acid | ||
| SMILES | CC(C)(C)NCC(C1=CC(=CC(=C1)O)O)O.CC(C)(C)NCC(C1=CC(=CC(=C1)O)O)O.OS(=O)(=O)O | ||
| Standard InChIKey | KFVSLSTULZVNPG-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/2C12H19NO3.H2O4S/c2*1-12(2,3)13-7-11(16)8-4-9(14)6-10(15)5-8;1-5(2,3)4/h2*4-6,11,13-16H,7H2,1-3H3;(H2,1,2,3,4) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||

Terbutaline Sulfate Dilution Calculator

Terbutaline Sulfate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.8227 mL | 9.1133 mL | 18.2266 mL | 36.4531 mL | 45.5664 mL |
| 5 mM | 0.3645 mL | 1.8227 mL | 3.6453 mL | 7.2906 mL | 9.1133 mL |
| 10 mM | 0.1823 mL | 0.9113 mL | 1.8227 mL | 3.6453 mL | 4.5566 mL |
| 50 mM | 0.0365 mL | 0.1823 mL | 0.3645 mL | 0.7291 mL | 0.9113 mL |
| 100 mM | 0.0182 mL | 0.0911 mL | 0.1823 mL | 0.3645 mL | 0.4557 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Terbutaline Sulfate is a selective β2-adrenergic receptor agonist with IC50 of 53 nM.
- Apelin-36 (rat, mouse)
Catalog No.:BCC5911
CAS No.:230299-95-3
- Physalin A
Catalog No.:BCN7920
CAS No.:23027-91-0
- Neoglycyrol
Catalog No.:BCN2907
CAS No.:23013-84-5
- MPTP hydrochloride
Catalog No.:BCC1778
CAS No.:23007-85-4
- Atazanavir sulfate (BMS-232632-05)
Catalog No.:BCC2114
CAS No.:229975-97-7
- AGN 194310
Catalog No.:BCC5416
CAS No.:229961-45-9
- GW9662
Catalog No.:BCC2260
CAS No.:22978-25-2
- Abn-CBD
Catalog No.:BCC7011
CAS No.:22972-55-0
- Desmethoxycentaureidin
Catalog No.:BCN5077
CAS No.:22934-99-2
- Neferine
Catalog No.:BCN6338
CAS No.:2292-16-2
- Ginkgolic acid C15:1
Catalog No.:BCN2307
CAS No.:22910-60-7
- R 892
Catalog No.:BCC5992
CAS No.:229030-05-1
- Z-β-Ala-OH
Catalog No.:BCC3058
CAS No.:2304-94-1
- Z-Asn-OH
Catalog No.:BCC2794
CAS No.:2304-96-3
- Z-Arg(NO2)-OH
Catalog No.:BCC3063
CAS No.:2304-98-5
- Ajugasterone C
Catalog No.:BCN2757
CAS No.:23044-80-6
- Lofepramine
Catalog No.:BCC7402
CAS No.:23047-25-8
- Dihydroconiferyl alcohol
Catalog No.:BCN7047
CAS No.:2305-13-7
- L-AP4
Catalog No.:BCC6550
CAS No.:23052-81-5
- (-)-Sophoranone
Catalog No.:BCN7162
CAS No.:23057-55-8
- Sinensetin
Catalog No.:BCN6356
CAS No.:2306-27-6
- Varenicline Hydrochloride
Catalog No.:BCC4156
CAS No.:230615-23-3
- Eurycomalactone
Catalog No.:BCN3108
CAS No.:23062-24-0
- PD 102807
Catalog No.:BCC7145
CAS No.:23062-91-1
Preparation and characterization of a sustained release buccoadhesive system for delivery of terbutaline sulfate.[Pubmed:24082891]
Res Pharm Sci. 2013 Oct;8(4):219-31.
Terbutaline Sulfate exhibits extensive first pass metabolism and a short elimination half life which makes frequent oral administration of the drug inevitable. A novel buccoadhesive controlled delivery system of the drug can easily overcome the problem. A two-layered core tablet composed of a fast release layer made of mannitol, lactose, PEG and the drug attached to a sustained release layer composed of drug, varying ratios of HPMC, Carbomer 934 (CP), and lactose capped with a buccoadhesive cup coated with an impermeable backing layer was developed. Buccoadhesive cup initially optimized for bioadhesion strength using HPMC and CP with various ratios. Drug transport through buccal membrane indicated a high permeability coefficient (0.00105 cm/sec). All tablets were acceptable with regard to drug contents, thickness, weight variations, hardness and drug content uniformity. The CP:HPMC 2:1 mixture showed the best mucoadhesion properties and was selected as excipient for the cup layer. Swelling index was higher for formulations containing greater amount of lactose and lower percentage of polymers. Fast release layer released its entire content within 15 min while sustained release layer lasted for 12 h. Drug release controlled by a combination of diffusion and chain relaxation mechanism.
Methacholine challenge tests to demonstrate therapeutic equivalence of terbutaline sulfate via different Turbuhaler((R)) devices in patients with mild to moderate asthma: Appraisal of a four-way crossover design.[Pubmed:28232118]
Pulm Pharmacol Ther. 2017 Jun;44:1-6.
BACKGROUND/OBJECTIVE: To demonstrate therapeutic equivalence of terbutaline via two different Turbuhaler((R)) devices by evaluating its protective effect against methacholine-induced bronchoconstriction in stable asthma. METHODS: In this double-blind, double-dummy, multicentre, single-dose, 4-way crossover study, patients with stable mild-to-moderate asthma (FEV1 >/=80% predicted) were randomised to 0.5 or 1.5 mg terbutaline via either Turbuhaler((R)) M2 or Turbuhaler((R)) M3 followed by a methacholine challenge test. The primary outcome variable was the concentration of methacholine causing a 20% drop in FEV1 (PC20). Patients had a PC20 methacholine <8 mg/mL that was reproducible after 2 weeks, and a stable baseline FEV1 at all visits (90-110% of enrolment value). RESULTS: 60 patients (mean age 31.1 years [range:18-64]; mean FEV1 92.1% predicted normal [78.4-120.6%]) were randomised to treatment; all completed the study. There was a clear dose-response for both devices. The within-device ratios (1.5 mg:0.5 mg) were 1.79 and 1.87 for Turbuhaler((R)) M3 and M2, respectively (both p < 0.001). The between-device ratios (M3:M2) were 0.92 (95% CI: 0.75-1.13) for 0.5 mg and 0.88 (95% CI 0.72-1.08) for 1.5 mg. Both confidence intervals lie inside the interval 0.67-1.50, which was the pre-specified condition for equivalent effect. CONCLUSIONS: Bronchoprotection using a standardised methacholine challenge model proved to be an effective design to elucidate therapeutic equivalence between devices in patients with mild-to-moderate asthma. The findings indicate that patients may switch from one type of Turbuhaler((R)) to the other without adjustment of therapy. Moreover, they show the robustness and utility of this study design and its suitability for investigating therapeutic equivalence. EUDRACT NUMBER: 2014-001457-16. CLINICALTRIALS. GOV IDENTIFIER: NCT02322788.
Binding studies of terbutaline sulfate to calf thymus DNA using multispectroscopic and molecular docking techniques.[Pubmed:26123508]
Spectrochim Acta A Mol Biomol Spectrosc. 2015;150:921-7.
The interaction of Terbutaline Sulfate (TS) with calf thymus DNA (ctDNA) were investigated by fluorescence quenching, UV-vis absorption, viscosity measurements, ionic strength effect, DNA melting experiments and molecular docking. The binding constants (Ka) of TS to ctDNA were determined as 4.92x10(4), 1.26x10(4) and 1.16x10(4) L mol(-1) at 17, 27 and 37 degrees C, respectively. Stern-Volmer plots suggested that the quenching of fluorescence of TS by ctDNA was a static quenching. The absorption spectra of TS with ctDNA revealed a slight blue shift and hyperchromic effect. The relative viscosity ctDNA was hardly changed by TS, and melting temperature varied slightly. For the system of TS-ctDNA, the intensity of fluorescence decreased with the increase of ionic strength. Also, the Ka for TS-double stranded DNA (dsDNA) was clearly weaker than that for TS-single stranded DNA (ssDNA). All these results revealed that the binding mode of TS with ctDNA should be groove binding. The enthalpy change and entropy change suggested that van der Waals force or hydrogen bonds was a main binding force between TS and ctDNA. Furthermore, the quantum yield of TS was measured by comparing with the standard solution. Based on the Forster energy transference theory (FRET), the binding distance between the acceptor and donor was calculated. Molecular docking showed that TS was a minor groove binder of ctDNA and preferentially bound to A-T rich regions.
Repurpose terbutaline sulfate for amyotrophic lateral sclerosis using electronic medical records.[Pubmed:25739475]
Sci Rep. 2015 Mar 5;5:8580.
Prediction of new disease indications for approved drugs by computational methods has been based largely on the genomics signatures of drugs and diseases. We propose a method for drug repositioning that uses the clinical signatures extracted from over 13 years of electronic medical records from a tertiary hospital, including >9.4 M laboratory tests from >530,000 patients, in addition to diverse genomics signatures. Cross-validation using over 17,000 known drug-disease associations shows this approach outperforms various predictive models based on genomics signatures and a well-known "guilt-by-association" method. Interestingly, the prediction suggests that Terbutaline Sulfate, which is widely used for asthma, is a promising candidate for amyotrophic lateral sclerosis for which there are few therapeutic options. In vivo tests using zebrafish models found that Terbutaline Sulfate prevents defects in axons and neuromuscular junction degeneration in a dose-dependent manner. A therapeutic potential of Terbutaline Sulfate was also observed when axonal and neuromuscular junction degeneration have already occurred in zebrafish model. Cotreatment with a beta2-adrenergic receptor antagonist, butoxamine, suggests that the effect of terbutaline is mediated by activation of beta2-adrenergic receptors.


