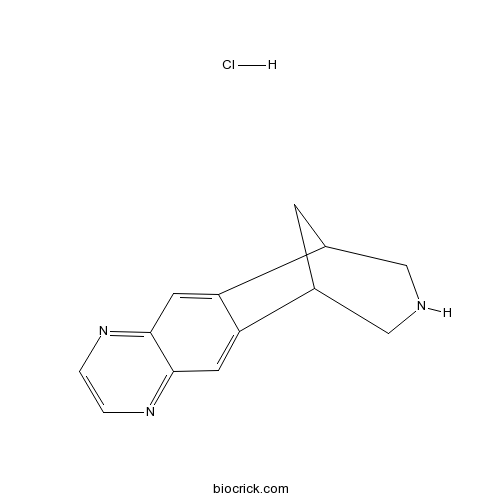
Varenicline Hydrochlorideα4β2 nicotinic receptor agonist CAS# 230615-23-3 |
- Olprinone
Catalog No.:BCC1820
CAS No.:106730-54-5
- Olprinone Hydrochloride
Catalog No.:BCC1821
CAS No.:119615-63-3
- Nortadalafil
Catalog No.:BCC1806
CAS No.:171596-36-4
- Apremilast (CC-10004)
Catalog No.:BCC2273
CAS No.:608141-41-9
- Oglemilast
Catalog No.:BCC1817
CAS No.:778576-62-8
- AN-2728
Catalog No.:BCC1361
CAS No.:906673-24-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 230615-23-3 | SDF | Download SDF |
| PubChem ID | 50878597 | Appearance | Powder |
| Formula | C13H14ClN3 | M.Wt | 247.72 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | CP 526555 hydrochloride; Champix hydrochloride; Chantix hydrochloride | ||
| Solubility | DMSO : ≥ 2.5 mg/mL (10.09 mM) *"≥" means soluble, but saturation unknown. | ||
| Chemical Name | 5,8,14-triazatetracyclo[10.3.1.02,11.04,9]hexadeca-2,4,6,8,10-pentaene;hydrochloride | ||
| SMILES | C1C2CNCC1C3=CC4=NC=CN=C4C=C23.Cl | ||
| Standard InChIKey | ZUCZFANFKYSVKF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H13N3.ClH/c1-2-16-13-5-11-9-3-8(6-14-7-9)10(11)4-12(13)15-1;/h1-2,4-5,8-9,14H,3,6-7H2;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Varenicline Hcl(CP 526555;Champix) is a nicotinic receptor partial agonist; it stimulates nicotine receptors more weakly than nicotine itself does.
IC50 value:
Target: nAChR
Varenicline(CP 526555; Champix; Chantix) is a prescription medication used to treat smoking addiction. As a partial agonist it both reduces cravings for and decreases the pleasurable effects of cigarettes and other tobacco products. Through these mechanisms Varenicline(CP 526555; Champix; Chantix) can assist some patients to quit smoking. References: | |||||

Varenicline Hydrochloride Dilution Calculator

Varenicline Hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.0368 mL | 20.1841 mL | 40.3682 mL | 80.7363 mL | 100.9204 mL |
| 5 mM | 0.8074 mL | 4.0368 mL | 8.0736 mL | 16.1473 mL | 20.1841 mL |
| 10 mM | 0.4037 mL | 2.0184 mL | 4.0368 mL | 8.0736 mL | 10.092 mL |
| 50 mM | 0.0807 mL | 0.4037 mL | 0.8074 mL | 1.6147 mL | 2.0184 mL |
| 100 mM | 0.0404 mL | 0.2018 mL | 0.4037 mL | 0.8074 mL | 1.0092 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Varenicline Hydrochloride is a partial agonist of α4β2 nicotinic receptor for smoking cessation [1].
Varenicline Hydrochloride has been reported to potently inhibit nAChR binding with the Ki values of 0.12±0.02 nM and 0.14±0.01 nM for α6β2* nAChR and α4β2* nAChR, respectively in rat striatum, 0.13±0.01 nM and 0.19±0.11 nM forα6β2* nAChR and α4β2* nAChR, respectively in monkey striatum. In addition, varenicline Hydrochloride has shown to be more potent in stimulating both α6β2* and α4β2* nAChRs compared with nicotine with the EC50 values of 0.007 and 0.086μM, respectively in rat striatum [1].
References:
[1] Bordia T1, Hrachova M, Chin M, McIntosh JM, Quik M. Varenicline is a potent partial agonist at α6β2* nicotinic acetylcholine receptors in rat and monkey striatum. J Pharmacol Exp Ther. 2012 Aug;342(2):327-34. doi: 10.1124/jpet.112.194852. Epub 2012 May 1.
- Sinensetin
Catalog No.:BCN6356
CAS No.:2306-27-6
- (-)-Sophoranone
Catalog No.:BCN7162
CAS No.:23057-55-8
- L-AP4
Catalog No.:BCC6550
CAS No.:23052-81-5
- Dihydroconiferyl alcohol
Catalog No.:BCN7047
CAS No.:2305-13-7
- Lofepramine
Catalog No.:BCC7402
CAS No.:23047-25-8
- Ajugasterone C
Catalog No.:BCN2757
CAS No.:23044-80-6
- Z-Arg(NO2)-OH
Catalog No.:BCC3063
CAS No.:2304-98-5
- Z-Asn-OH
Catalog No.:BCC2794
CAS No.:2304-96-3
- Z-β-Ala-OH
Catalog No.:BCC3058
CAS No.:2304-94-1
- Terbutaline Sulfate
Catalog No.:BCC4320
CAS No.:23031-32-5
- Apelin-36 (rat, mouse)
Catalog No.:BCC5911
CAS No.:230299-95-3
- Physalin A
Catalog No.:BCN7920
CAS No.:23027-91-0
- Eurycomalactone
Catalog No.:BCN3108
CAS No.:23062-24-0
- PD 102807
Catalog No.:BCC7145
CAS No.:23062-91-1
- 4-Amino-N-methylphthalimide
Catalog No.:BCC8686
CAS No.:2307-00-8
- Xylazine HCl
Catalog No.:BCC4341
CAS No.:23076-35-9
- Sitosteryl palmitate
Catalog No.:BCN5078
CAS No.:2308-85-2
- 2-amino-3-(3-bromo-5-chloro-4-hydroxyphenyl)propanoic acid
Catalog No.:BCN8284
CAS No.:
- Corilagin
Catalog No.:BCN2322
CAS No.:23094-69-1
- Chebulagic acid
Catalog No.:BCN3262
CAS No.:23094-71-5
- Neuropeptide SF (mouse, rat)
Catalog No.:BCC6054
CAS No.:230960-31-3
- UK 356618
Catalog No.:BCC2378
CAS No.:230961-08-7
- UK 370106
Catalog No.:BCC2379
CAS No.:230961-21-4
- Fumagillin
Catalog No.:BCC2347
CAS No.:23110-15-8
"Impact of Smoking Cessation Treatment" on Lung Function and Response Rate in EGFR Mutated Patients: A Short-Term Cohort Study.[Pubmed:26246248]
Recent Pat Anticancer Drug Discov. 2015;10(3):342-51.
BACKGROUND: Erlotinib is a validated drug "for the treatment of patients affected by advanced unresectable non small cell lung cancer (NSCLC) with EGFR mutations". We want to focus on potential functional benefits deriving from a combined therapy containing TKI (erlotinib) and a nicotinic partial agonist (varenicicline) in smokers. METHODS: we analyzed the records of patients affected by NSCLC treated undergoing "first line therapy with Erlotinib" and smoking cessation (with varenicicline). Response to therapy was evaluated by CT scan. Data concerning clinical history, smoking habit, nicotine dependence were collected after three months from the beginning of the recruitment. Pulmonary function tests including spirometry with pletismographic technique and exhaled carbon monoxide (CO) were performed with recording of resistances, flows, volumes. A group of ten current smokers affected by NSCLC with EGFR activating mutation and concurrent mild COPD undergoing anti-EGFR treatment without smoking cessation was used to compare clinical and functional data. A control group of NSCLC wild type with mild COPD undergoing smoking cessation was assessed for functional data. RESULTS: Twenty-five patients were enrolled. All of them reported partial response at CT re-evaluation. All functional indexes and parameters were improved after combined treatment a significant increase of FEV1 level and a decrease of exhaled CO. In particular, a mean increase of FEV1 from 1.93 (SD 0.48) to 2.03(SD 0.46) liters was recorded. A notable reduction of sRAW (specific resistances) was also observed. The improvement of some parameters such as CO, heart rate (HR), sRAW and FEV1 resulted statistically significant. A better response to therapy was found "in the study group compared to the second group of mutated NSCLC patients". In this second group, we also observed an improvement of functional obstructive parameters although it was less remarkable than study group. Only sRAW and FEF 25/75 were significantly increased. The group of NSCLC wild type undergoing only smoking cessation showed a lower increase of FEV1 by about 50 ml compared to the first group. CONCLUSION: The combination of anti-EGFR treatment and concurrent therapy for smoking cessation seems to be more effective than erlotinib alone in improving lung function and clinical response in advanced NSCLC patients with EGFR-mutations. It is conceivable that erlotinib may potentiate the action of varenicline. We have also outlined some relevant patents concerning varenicline and EGFR-TKI.
Neuroimaging the Effectiveness of Substance Use Disorder Treatments.[Pubmed:27184387]
J Neuroimmune Pharmacol. 2016 Sep;11(3):408-33.
Neuroimaging techniques to measure the function and biochemistry of the human brain such as positron emission tomography (PET), proton magnetic resonance spectroscopy ((1)H MRS), and functional magnetic resonance imaging (fMRI), are powerful tools for assessing neurobiological mechanisms underlying the response to treatments in substance use disorders. Here, we review the neuroimaging literature on pharmacological and behavioral treatment in substance use disorder. We focus on neural effects of medications that reduce craving (e.g., naltrexone, bupropion hydrochloride, baclofen, methadone, varenicline) and that improve cognitive control (e.g., modafinil, N-acetylcysteine), of behavioral treatments for substance use disorders (e.g., cognitive bias modification training, virtual reality, motivational interventions) and neuromodulatory interventions such as neurofeedback and transcranial magnetic stimulation. A consistent finding for the effectiveness of therapeutic interventions identifies the improvement of executive control networks and the dampening of limbic activation, highlighting their values as targets for therapeutic interventions in substance use disorders.
American Psychiatric Association - 168th Annual Meeting (May 16-20, 2015 - Toronto, Canada).[Pubmed:26261851]
Drugs Today (Barc). 2015 Jun;51(6):375-82.
The theme of this year's American Psychiatric Association (APA) meeting was 'Psychiatry: integrating body and mind, heart and soul', with special focus given to advances in basic and cognitive neuroscience and how these may contribute to integrated care of mental health and illness. The program featured numerous tracks and subtracks in areas of interest such as addiction psychiatry, child, adolescent and geriatric psychiatry, and psychosomatic medicine.
Emerging drugs for the treatment of tobacco dependence: 2014 update.[Pubmed:24654737]
Expert Opin Emerg Drugs. 2014 Jun;19(2):243-60.
INTRODUCTION: Tobacco dependence remains a global epidemic and the largest preventable cause of morbidity and mortality around the world. Smoking cessation has benefits at all ages but remains challenging for several reasons, among which are the complexities of nicotine addiction and limitations of available pharmacotherapies. AREAS COVERED: This review summarizes current and emerging pharmacotherapies for the treatment of tobacco dependence, including first- and second-line recommended agents. Medications with alternative primary indications that have been investigated as potential treatments for tobacco dependence are also discussed. Articles reviewed were obtained through searches of PubMed, Ovid MEDLINE, ClinicalTrials.gov and the Pharmaprojects database. EXPERT OPINION: Current evidence suggests that the two most effective pharmacotherapies to treat tobacco dependence are varenicline and combination nicotine replacement therapy. Alternative agents investigated demonstrate mixed rates of success in achieving long-term abstinence from smoking. No single pharmacotherapy will serve as a universally successful treatment given the complex underpinnings of tobacco dependence and individuality of smokers. The ultimate goal of tobacco research with respect to pharmacotherapeutic development continues to be providing clinicians with an armamentarium of drugs to choose from allowing for tailoring of treatment for smokers.


