SinensetinCAS# 2306-27-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2306-27-6 | SDF | Download SDF |
| PubChem ID | 145659 | Appearance | White-pale yellow powder |
| Formula | C20H20O7 | M.Wt | 372.37 |
| Type of Compound | Flavonoids | Storage | Desiccate at -20°C |
| Synonyms | Pedalitin permethyl ether | ||
| Solubility | DMSO : 12.5 mg/mL (33.57 mM; Need ultrasonic) | ||
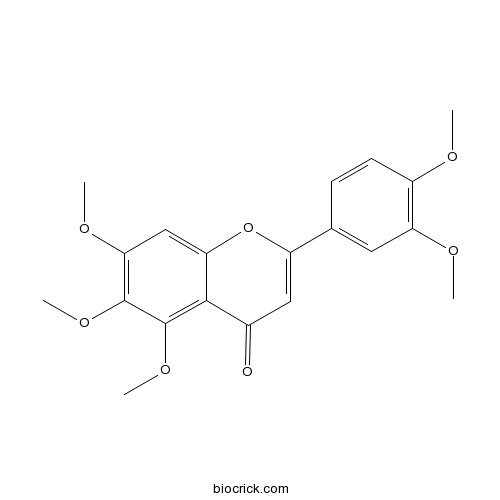
| Chemical Name | 2-(3,4-dimethoxyphenyl)-5,6,7-trimethoxychromen-4-one | ||
| SMILES | COC1=C(C=C(C=C1)C2=CC(=O)C3=C(C(=C(C=C3O2)OC)OC)OC)OC | ||
| Standard InChIKey | LKMNXYDUQXAUCZ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H20O7/c1-22-13-7-6-11(8-15(13)23-2)14-9-12(21)18-16(27-14)10-17(24-3)19(25-4)20(18)26-5/h6-10H,1-5H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Sinensetin is a polymethoxylated flavonoids in citrus fruit, as a novel antiangiogenesis agent, has potential for anti-carcinogenesis, antitumor, and cardiovascular protective activities. Sinensetin has antioxidative effect and has anti-inflammatory by regulating the protein level of inhibitorκB-α, it enhances adipogenesis and lipolysis by increasing cAMP levels in adipocytes. |
| Targets | cAMP | AMPK | NOS | COX | NO | PGE | TNF-α | STAT | IkB | IKK |
| In vitro | Sinensetin Enhances Adipogenesis and Lipolysis by Increasing Cyclic Adenosine Monophosphate Levels in 3T3-L1 Adipocytes.[Pubmed: 25735898]Biol Pharm Bull. 2015 Apr 1;38(4):552-8.Sinensetin is a rare polymethoxylated flavone (PMF) found in certain citrus fruits.
|
| In vivo | Flavonoids eupatorin and sinensetin present in Orthosiphon stamineus leaves inhibit inflammatory gene expression and STAT1 activation.[Pubmed: 22516932]Planta Med. 2012 May;78(8):779-86.Cytokines and other inflammatory mediators, such as prostaglandin E₂ (PGE₂) and nitric oxide (NO) produced by cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), respectively, activate and drive inflammation and therefore serve as targets for anti-inflammatory drug development. Orthosiphon stamineus is an indigenous medicinal plant of Southeast Asia that has been traditionally used in the treatment of rheumatoid arthritis, gout, and other inflammatory disorders.
|
| Animal Research | In vitro and in vivo structure and activity relationship analysis of polymethoxylated flavonoids: identifying sinensetin as a novel antiangiogenesis agent.[Pubmed: 22707269]Mol Nutr Food Res. 2012 Jun;56(6):945-56.Polymethoxylated flavonoids are present in citrus fruit in a range of chemical structures and abundance. These compounds have potential for anticarcinogenesis, antitumor, and cardiovascular protective activity, but the effect on angiogenesis has not been well studied.
|
| Structure Identification | Food Chem., 2009, 113(1): 185-90.Sinensetin, rutin, 3'-hydroxy-5, 6, 7, 4'-tetramethoxyflavone and rosmarinic acid contents and antioxidative effect of the skin of apple fruit[Reference: WebLink]
|

Sinensetin Dilution Calculator

Sinensetin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6855 mL | 13.4275 mL | 26.855 mL | 53.71 mL | 67.1375 mL |
| 5 mM | 0.5371 mL | 2.6855 mL | 5.371 mL | 10.742 mL | 13.4275 mL |
| 10 mM | 0.2686 mL | 1.3428 mL | 2.6855 mL | 5.371 mL | 6.7138 mL |
| 50 mM | 0.0537 mL | 0.2686 mL | 0.5371 mL | 1.0742 mL | 1.3428 mL |
| 100 mM | 0.0269 mL | 0.1343 mL | 0.2686 mL | 0.5371 mL | 0.6714 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Sinensetin is a methylated flavone found in certain citrus fruits. pocess potent antiangiogenesis and anti-inflammatory, sinensetin enhances adipogenesis and lipolysis. In vitro: Sinensetin promots adipogenesis in 3T3-L1 preadipocytes growing in incomplete differentiation medium, sinensetin enhances adipogenesis and lipolysis by increasing cAMP levels. [1] Sinensetin shows anti-inflammatory activity by regulating the protein level of inhibitor κB-α (IκB-α). [2] In vivo: Sinensetin has the most potent antiangiogenesis activity and the lowest toxicity, inhibits angiogenesis by inducing cell cycle arrest in the G0/G1 phase in HUVEC culture and downregulating the mRNA expressions of angiogenesis genes flt1, kdrl, and hras in zebrafish. [3]
References:
[1]. Kang SI et al. Sinensetin enhances adipogenesis and lipolysis by increasing cyclic adenosine monophosphate levels in 3T3-L1 adipocytes. Biol Pharm Bull. 2015;38(4):552-8.
[2]. Shin HS et al. Sinensetin attenuates LPS-induced inflammation by regulating the protein level of IκB-α. Biosci Biotechnol Biochem. 2012;76(4):847-9.
[3]. Lam IK et al. In vitro and in vivo structure and activity relationship analysis of polymethoxylated flavonoids: identifying sinensetin as a novel antiangiogenesis agent. Mol Nutr Food Res. 2012 Jun;56(6):945-56.
- (-)-Sophoranone
Catalog No.:BCN7162
CAS No.:23057-55-8
- L-AP4
Catalog No.:BCC6550
CAS No.:23052-81-5
- Dihydroconiferyl alcohol
Catalog No.:BCN7047
CAS No.:2305-13-7
- Lofepramine
Catalog No.:BCC7402
CAS No.:23047-25-8
- Ajugasterone C
Catalog No.:BCN2757
CAS No.:23044-80-6
- Z-Arg(NO2)-OH
Catalog No.:BCC3063
CAS No.:2304-98-5
- Z-Asn-OH
Catalog No.:BCC2794
CAS No.:2304-96-3
- Z-β-Ala-OH
Catalog No.:BCC3058
CAS No.:2304-94-1
- Terbutaline Sulfate
Catalog No.:BCC4320
CAS No.:23031-32-5
- Apelin-36 (rat, mouse)
Catalog No.:BCC5911
CAS No.:230299-95-3
- Physalin A
Catalog No.:BCN7920
CAS No.:23027-91-0
- Neoglycyrol
Catalog No.:BCN2907
CAS No.:23013-84-5
- Varenicline Hydrochloride
Catalog No.:BCC4156
CAS No.:230615-23-3
- Eurycomalactone
Catalog No.:BCN3108
CAS No.:23062-24-0
- PD 102807
Catalog No.:BCC7145
CAS No.:23062-91-1
- 4-Amino-N-methylphthalimide
Catalog No.:BCC8686
CAS No.:2307-00-8
- Xylazine HCl
Catalog No.:BCC4341
CAS No.:23076-35-9
- Sitosteryl palmitate
Catalog No.:BCN5078
CAS No.:2308-85-2
- 2-amino-3-(3-bromo-5-chloro-4-hydroxyphenyl)propanoic acid
Catalog No.:BCN8284
CAS No.:
- Corilagin
Catalog No.:BCN2322
CAS No.:23094-69-1
- Chebulagic acid
Catalog No.:BCN3262
CAS No.:23094-71-5
- Neuropeptide SF (mouse, rat)
Catalog No.:BCC6054
CAS No.:230960-31-3
- UK 356618
Catalog No.:BCC2378
CAS No.:230961-08-7
- UK 370106
Catalog No.:BCC2379
CAS No.:230961-21-4
Sinensetin enhances adipogenesis and lipolysis by increasing cyclic adenosine monophosphate levels in 3T3-L1 adipocytes.[Pubmed:25735898]
Biol Pharm Bull. 2015;38(4):552-8.
Sinensetin is a rare polymethoxylated flavone (PMF) found in certain citrus fruits. In this study, we investigated the effects of Sinensetin on lipid metabolism in 3T3-L1 cells. Sinensetin promoted adipogenesis in 3T3-L1 preadipocytes growing in incomplete differentiation medium, which did not contain 3-isobutyl-1-methylxanthine. Sinensetin up-regulated expression of the adipogenic transcription factors peroxisome proliferator-activated receptor gamma, CCAAT/enhancer-binding protein (C/EBP) alpha, and sterol regulatory element-binding protein 1c. It also potentiated expression of C/EBPbeta and activation of cAMP-responsive element-binding protein. Sinensetin enhanced activation of protein kinase A and increased intracellular cAMP levels in 3T3-L1 preadipocytes. In mature 3T3-L1 adipocytes, Sinensetin stimulated lipolysis via a cAMP pathway. Taken together, these results suggest that Sinensetin enhances adipogenesis and lipolysis by increasing cAMP levels in adipocytes.
Flavonoids eupatorin and sinensetin present in Orthosiphon stamineus leaves inhibit inflammatory gene expression and STAT1 activation.[Pubmed:22516932]
Planta Med. 2012 May;78(8):779-86.
Cytokines and other inflammatory mediators, such as prostaglandin E(2) (PGE(2)) and nitric oxide (NO) produced by cyclooxygenase-2 (COX-2) and inducible nitric oxide synthase (iNOS), respectively, activate and drive inflammation and therefore serve as targets for anti-inflammatory drug development. Orthosiphon stamineus is an indigenous medicinal plant of Southeast Asia that has been traditionally used in the treatment of rheumatoid arthritis, gout, and other inflammatory disorders. The present study investigated the anti-inflammatory properties of Orthosiphon stamineus leaf chloroform extract (CE), its flavonoid-containing CE fraction 2 (CF2), and the flavonoids eupatorin, eupatorin-5-methyl ether (TMF), and Sinensetin, identified from the CF2. It was found that CE (20 and 50 microg/mL) and CF2 (20 and 50 microg/mL) inhibited iNOS expression and NO production, as well as PGE(2) production. Eupatorin and Sinensetin inhibited iNOS and COX-2 expression and the production of NO (IC(5)(0) 5.2 microM and 9.2 microM for eupatorin and Sinensetin, respectively) and PGE(2) (IC(5)(0) 5.0 microM and 2.7 microM for eupatorin and Sinensetin, respectively) in a dose-dependent manner. The extracts and the compounds also inhibited tumor necrosis factor alpha (TNF-alpha) production (IC(5)(0) 5.0 microM and 2.7 microM for eupatorin and Sinensetin, respectively). Eupatorin and Sinensetin inhibited lipopolysaccharide (LPS)-induced activation of transcription factor signal transducers and activators of transcription 1alpha (STAT1alpha). Furthermore, eupatorin (50 mg/kg i. p.) and Sinensetin (50 mg/kg i. p.) inhibited carrageenan-induced paw inflammation in mice. The results suggest that CE and CF2, as well as the known constituents of CF2, i.e., eupatorin and Sinensetin, have meaningful anti-inflammatory properties which may be utilized in the development of novel anti-inflammatory treatments.
In vitro and in vivo structure and activity relationship analysis of polymethoxylated flavonoids: identifying sinensetin as a novel antiangiogenesis agent.[Pubmed:22707269]
Mol Nutr Food Res. 2012 Jun;56(6):945-56.
SCOPE: Polymethoxylated flavonoids are present in citrus fruit in a range of chemical structures and abundance. These compounds have potential for anticarcinogenesis, antitumor, and cardiovascular protective activity, but the effect on angiogenesis has not been well studied. METHODS AND RESULTS: Human umbilical vein endothelial cells (HUVECs) in vitro and zebrafish (Danio rerio) in vivo models were used to screen and identify the antiangiogenesis activity of seven polymethoxylated flavonoids; namely, hesperetin, naringin, neohesperidin, nobiletin, scutellarein, scutellarein tetramethylether, and Sinensetin. Five, excluding naringin and neohesperidin, showed different degrees of potency of antiangiogenesis activity. Sinensetin, which had the most potent antiangiogenesis activity and the lowest toxicity, inhibited angiogenesis by inducing cell cycle arrest in the G0/G1 phase in HUVEC culture and downregulating the mRNA expressions of angiogenesis genes flt1, kdrl, and hras in zebrafish. CONCLUSION: The in vivo structure-activity relationship (SAR) analysis indicated that a flavonoid with a methoxylated group at the C3' position offers a stronger antiangiogenesis activity, whereas the absence of a methoxylated group at the C8 position offers lower lethal toxicity in addition to enhancing the antiangiogenesis activity. This study provides new insight into how modification of the chemical structure of polymethoxylated flavonoids affects this newly identified antiangiogenesis activity.
Sinensetin attenuates LPS-induced inflammation by regulating the protein level of IkappaB-alpha.[Pubmed:22484952]
Biosci Biotechnol Biochem. 2012;76(4):847-9.
Sinensetin is one of the polymethoxyflavones (PMFs) having five methoxy groups on the basic benzo-gamma-pyrone skeleton with a carbonyl group at the C(4) position. We investigated in this study the anti-inflammatory activity of Sinensetin in lipopolysaccharide (LPS)-stimulated RAW 264.7 cells. Sinensetin showed anti-inflammatory activity by regulating the protein level of inhibitor kappaB-alpha (IkappaB-alpha).
Effects of sinensetin on lipid metabolism in mature 3T3-L1 adipocytes.[Pubmed:22438091]
Phytother Res. 2013 Jan;27(1):131-4.
Sinensetin is a rare polymethoxylated flavone found in certain citrus fruits. In this study, we investigated the effects of Sinensetin on lipid metabolism in mature 3T3-L1 adipocytes. Sinensetin decreased the expression of sterol regulatory element-binding protein 1c (SREBP1c), suggesting its antiadipogeneic property via downreguation of SREBP1c. Also, Sinensetin increased the phosphorylation of protein kinase A and hormone-sensitive lipase, indicating its lipolytic property via a cAMP-mediated signaling pathway. Moreover, Sinensetin inhibited insulin-stimulated glucose uptake by decreasing the phosphorylation of insulin receptor substrate and Akt. Furthermore, Sinensetin increased the phosphorylation of AMP-activated protein kinase (AMPK) and acetyl-CoA carboxylase. It also upregulated mRNA expression of carnitine palmitoyltransferase-1a, suggesting that Sinensetin enhances fatty acid beta-oxidation through the AMPK pathway. Taken together, these results suggest that Sinensetin may have potential as a natural agent for prevention/improvement of obesity.


