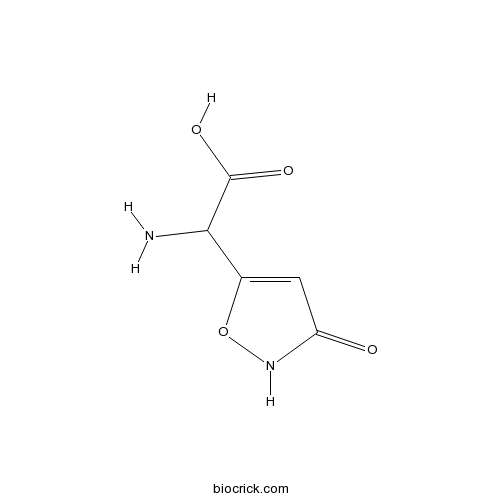
Ibotenic acidNon-selective mGlu agonist, also NMDA agonist CAS# 2552-55-8 |
- I-BET-762
Catalog No.:BCC4474
CAS No.:1260907-17-2
- MS436
Catalog No.:BCC4037
CAS No.:1395084-25-9
- PFI-1 (PF-6405761)
Catalog No.:BCC2225
CAS No.:1403764-72-6
- SGC-CBP30
Catalog No.:BCC2419
CAS No.:1613695-14-9
- Bromosporine
Catalog No.:BCC2226
CAS No.:1619994-69-2
- OTX-015
Catalog No.:BCC1829
CAS No.:202590-98-5
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 2552-55-8 | SDF | Download SDF |
| PubChem ID | 1233 | Appearance | Powder |
| Formula | C5H6N2O4 | M.Wt | 158.11 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | H2O : 6 mg/mL (37.95 mM; Need ultrasonic and warming) | ||
| Chemical Name | 2-amino-2-(3-oxo-1,2-oxazol-5-yl)acetic acid | ||
| SMILES | C1=C(ONC1=O)C(C(=O)O)N | ||
| Standard InChIKey | IRJCBFDCFXCWGO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C5H6N2O4/c6-4(5(9)10)2-1-3(8)7-11-2/h1,4H,6H2,(H,7,8)(H,9,10) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | NMDA and metabotropic glutamate receptor agonist. |

Ibotenic acid Dilution Calculator

Ibotenic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.3247 mL | 31.6236 mL | 63.2471 mL | 126.4942 mL | 158.1178 mL |
| 5 mM | 1.2649 mL | 6.3247 mL | 12.6494 mL | 25.2988 mL | 31.6236 mL |
| 10 mM | 0.6325 mL | 3.1624 mL | 6.3247 mL | 12.6494 mL | 15.8118 mL |
| 50 mM | 0.1265 mL | 0.6325 mL | 1.2649 mL | 2.5299 mL | 3.1624 mL |
| 100 mM | 0.0632 mL | 0.3162 mL | 0.6325 mL | 1.2649 mL | 1.5812 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Bruceine A
Catalog No.:BCC5311
CAS No.:25514-31-2
- Bruceine C
Catalog No.:BCN8000
CAS No.:25514-30-1
- Bruceine B
Catalog No.:BCN7615
CAS No.:25514-29-8
- 1-Acetyl-4-piperidinecarboxylic acid
Catalog No.:BCC8447
CAS No.:25503-90-6
- 4-Phenylbutan-2-one
Catalog No.:BCN3808
CAS No.:2550-26-7
- 3-Epioleanolic acid
Catalog No.:BCN3050
CAS No.:25499-90-5
- Tasquinimod
Catalog No.:BCC1987
CAS No.:254964-60-8
- 4-Allyloxy-2-hydroxybenzophenone
Catalog No.:BCC8675
CAS No.:2549-87-3
- Kushenol X
Catalog No.:BCN3350
CAS No.:254886-77-6
- Kushenol W
Catalog No.:BCN3307
CAS No.:254886-76-5
- 2,3-Bis(3,4-dimethoxybenzyl)butyrolactone
Catalog No.:BCN1473
CAS No.:25488-59-9
- Curryangine
Catalog No.:BCN7907
CAS No.:25488-37-3
- Isoferulic acid
Catalog No.:BCN5122
CAS No.:25522-33-2
- Z-Ile-Glu-Pro-Phe-Ome
Catalog No.:BCC5526
CAS No.:255257-97-4
- Mayumbine
Catalog No.:BCN5123
CAS No.:25532-45-0
- Propidium iodide
Catalog No.:BCC8015
CAS No.:25535-16-4
- 1-(4-Hydroxybenzoyl)glucose
Catalog No.:BCN6900
CAS No.:25545-07-7
- 7-Methoxy-4-methylcoumarin
Catalog No.:BCN6540
CAS No.:2555-28-4
- Efetaal
Catalog No.:BCN8494
CAS No.:2556-10-7
- 1,6,7-Trihydroxyxanthone
Catalog No.:BCN5124
CAS No.:25577-04-2
- Delta-Tocotrienol
Catalog No.:BCN6696
CAS No.:25612-59-3
- 7beta-Acetoxytaxuspine C
Catalog No.:BCN7219
CAS No.:256347-91-8
- BAY 41-2272
Catalog No.:BCC7932
CAS No.:256376-24-6
- SEW 2871
Catalog No.:BCC7312
CAS No.:256414-75-2
Intrahippocampal Administration of Ibotenic Acid Induced Cholinergic Dysfunction via NR2A/NR2B Expression: Implications of Resveratrol against Alzheimer Disease Pathophysiology.[Pubmed:27199654]
Front Mol Neurosci. 2016 Apr 26;9:28.
Although several drugs revealed moderate amelioration of symptoms, none of them have sufficient potency to prevent or reverse the progression toward Alzheimer's disease (AD) pathology. Resveratrol (RSV), a polyphenolic compound has shown an outstanding therapeutic effect on a broad spectrum of diseases like age-associated neurodegeneration, inflammation etc. The present study was thus conducted to assess the therapeutic efficacy of RSV in ameliorating the deleterious effects of Ibotenic acid (IBO) in male Wistar rats. Stereotactic intrahippocampal administration of IBO (5 mug/mul) lesioned rats impairs cholinergic transmission, learning and memory performance that is rather related to AD and thus chosen as a suitable model to understand the drug efficacy in preventing AD pathophysiology. Since IBO is an agonist of glutamate, it is expected to exhibit an excitotoxic effect by altering glutamatergic receptors like NMDA receptor. The current study displayed significant alterations in the mRNA expression of NR2A and NR2B subunits of NMDA receptors, and further it is surprising to note that cholinergic receptors decreased in expression particularly alpha7-nAChR with increased m1AChR. RSV administration (20 mg/kg body weight, i.p.) significantly reduced these changes in IBO induced rats. Glutamatergic and cholinergic receptor alterations were associated with significant changes in the behavioral parameters of rats induced by IBO. While RSV improved spatial learning performance, attenuated immobility, and improvised open field activity in IBO induced rats. NR2B activation in the present study might mediate cell death through oxidative stress that form the basis of abnormal behavioral pattern in IBO induced rats. Interestingly, RSV that could efficiently encounter oxidative stress have significantly decreased stress markers viz., nitrite, PCO, and MDA levels by enhancing antioxidant status. Histopathological analysis displayed significant reduction in the hippocampal pyramidal layer thickness and live neurons in IBO induced rats, with slight pathological changes in the entorhinal cortex (EC) of rat brain, which was prevented on RSV administration. Our study thus concludes that RSV administration significantly ameliorated the deleterious effects in the IBO lesioned rat model for AD by alleviating cholinergic pathways, reducing oxidative stress and thereby improving spatial memory.
Icariin, a major constituent from Epimedium brevicornum, attenuates ibotenic acid-induced excitotoxicity in rat hippocampus.[Pubmed:27368415]
Behav Brain Res. 2016 Oct 15;313:111-119.
Excitotoxicity is one of the most extensively studied causes of neuronal death and plays an important role in Alzheimer's disease (AD). Icariin is a flavonoid component of a traditional Chinese medicine reported to possess a broad spectrum of pharmacological effects. The present study was designed to investigate the effects of icariin against learning and memory impairment induced by excitotoxicity. Here, we demonstrated that rats receiving intracerebroventricular injection of excitatory neurotoxin Ibotenic acid exhibited impaired learning and memory. Oral administration of icariin at doses of 20 and 40mg/kg rescued behavioral performance and protected against neurotoxicity in rat hippocampus by suppressing Ibotenic acid induced pro-apoptosis. Furthermore, Western blott of hippocampal specimens revealed that icariin up-regulated the expression of calbindin-D28k protein following Ibotenic acid administration. Additionally, icariin inhibited mitogen-activated protein kinase (MAPK) family phosphorylation and nuclear factor kappa B (NF-kappaB) signaling, implicating the MAPK signaling and NF-kappaB signaling pathways were involved in the mechanism underlying icariin-mediated neuroprotection against Ibotenic acid-induced excitotoxicity. These data suggested that icariin could be a potential agent for treatment of excitotoxicity-related diseases, including AD.
Alleviating effects of Bushen-Yizhi formula on ibotenic acid-induced cholinergic impairments in rat.[Pubmed:25482164]
Rejuvenation Res. 2015 Apr;18(2):111-27.
This study explored the curative effect and underlying mechanisms of a traditional Chinese medicine compound prescription, Bushen-Yizhi formula (BSYZ), in Ibotenic acid (IBO)-induced rats. Morris water maze and novel object recognition tests showed that BSYZ significantly improved spatial and object memory. Brain immunohistochemistry staining showed that BSYZ significantly up-regulated expression of choline acetyltransferase (ChAT) and nerve growth factor (NGF) in the hippocampus and cortex. The protein tyrosine kinase high-affinity receptor TrkA was slightly increased in the hippocampus and cortex, and significantly enhanced in the nucleus basalis of Meynert (NBM) after BSYZ intervention. The immunoreactivity of the p75 low-affinity receptor in BSYZ-treated rats was significantly strengthened in the cortex. Similar expression trends of nerve growth factor (NGF), TrkA, and p75 mRNA were observed in the hippocampus and cortex. Additionally, BSYZ reversed IBO-induced disorders of acetylcholine (ACh) levels, ChAT, and cholinesterase (ChE) in the cortex, which was consistent with the changes in mRNA levels of ChAT and acetylcholinesterase (AChE). Expression of ChAT and AChE proteins and mRNA in the hippocampus was up-regulated, whereas the apoptosis-relative protein cleaved caspase-3 was decreased after administration of BSYZ. Moreover, changes in cell death were confirmed by histological morphology. Thus, the results indicated that the BSYZ formula could ameliorate memory impairments in IBO-induced rats, and it exerted its therapeutic action probably by modulating cholinergic pathways, NGF signaling, and anti-apoptosis. Overall, it is suggested that the BSYZ formula might be a potential therapeutic approach for the treatment of Alzheimer's disease (AD) and other cholinergic impairment-related diseases.
Catching flies with Amanita muscaria: traditional recipes from Slovenia and their efficacy in the extraction of ibotenic acid.[Pubmed:27063872]
J Ethnopharmacol. 2016 Jul 1;187:1-8.
ETHNOPHARMACOLOGICAL RELEVANCE: Fly control is necessary for maintaining good hygiene on farms. Because organic farmers are skeptical about chemical pesticides, alternative fly control remedies are being considered. Amanita muscaria is a widespread fungus that contains Ibotenic acid and muscimol. This fungus has been used to catch flies for centuries, but traditional recipes are poorly described, documented and characterized. AIM OF THE STUDY: The aim of the present study was to collect the traditional methods for preparing A. muscaria for catching flies in Karst and Gorjanci and to investigate the influence of different traditional methods on the release of Ibotenic acid and muscimol from the fungal material. MATERIALS AND METHODS: The research was conducted in villages in Karst and in the foothills of Gorjanci, Slovenia. Data regarding the traditional recipes of A. muscaria for catching flies were collected through structured interviews with 31 people in Karst and 28 in Gorjanci. Eight preparations were prepared based on traditional methods, and the amount of Ibotenic acid and muscimol released from the fungal material at five different time points (0.5, 1, 2, 3 and 24h) was determined by high-performance liquid chromatography (HPLC). RESULTS: Detailed descriptions of preparations used for catching flies were obtained from three informants in Karst, who were originally from other parts of Slovenia, and 13 informants in the foothills of Gorjanci. However, there were no reports regarding current usage. A total of 9 different methods were collected. Some methods were simple and included soaking in milk or water or dripping a little milk onto the mushroom. Others were more complex and included a combination of heat or mechanical processing and soaking in milk or water. For all preparations, the release of Ibotenic acid was time-dependent, with the extracted amount increasing over time. Although milk was used more often than water in the traditional recipes, the release of both substances was not dependent on the solvent used. Fungal material that was exclusively soaked in water or milk released the smallest amount of Ibotenic acid and muscimol at each time point. Additional heat and mechanical processing led to faster release of Ibotenic acid and muscimol from the fungal material. CONCLUSIONS: The tradition of using A. muscaria for catching flies was present in Gorjanci but not in Karst. The methods used to prepare the fungal material vary, and these differences are reflected in the release profile of Ibotenic acid.
The metabotropic glutamate receptors: structure and functions.[Pubmed:7623957]
Neuropharmacology. 1995 Jan;34(1):1-26.
Glutamate is the main excitatory neurotransmitter in the brain. For many years it has been considered to act only on ligand-gated receptor channels--termed NMDA, AMPA and kainate receptors--involved in the fast excitatory synaptic transmission. Recently, glutamate has been shown to regulate ion channels and enzymes producing second messengers via specific receptors coupled to G-proteins. The existence of these receptors, called metabotropic glutamate receptors, is changing our views on the functioning of fast excitatory synapses.
Molecular diversity of glutamate receptors and implications for brain function.[Pubmed:1329206]
Science. 1992 Oct 23;258(5082):597-603.
The glutamate receptors mediate excitatory neurotransmission in the brain and are important in memory acquisition, learning, and some neurodegenerative disorders. This receptor family is classified in three groups: the N-methyl-D-aspartate (NMDA), alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionate (AMPA)-kainate, and metabotropic receptors. Recent molecular studies have shown that many receptor subtypes exist in all three groups of the receptors and exhibit heterogeneity in function and expression patterns. This article reviews the molecular and functional diversity of the glutamate receptors and discusses their implications for integrative brain function.


