KetanserinCAS# 74050-98-9 |
- Rocilinostat (ACY-1215)
Catalog No.:BCC2144
CAS No.:1316214-52-4
- LY 294002
Catalog No.:BCC3659
CAS No.:154447-36-6
- (±)-Bay K 8644
Catalog No.:BCC3918
CAS No.:71145-03-4
- Omeprazole
Catalog No.:BCC1254
CAS No.:73590-58-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 74050-98-9 | SDF | Download SDF |
| PubChem ID | 3822 | Appearance | Powder |
| Formula | C22H22FN3O3 | M.Wt | 395.43 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | R41468 | ||
| Solubility | DMSO : 16.67 mg/mL (42.16 mM; Need ultrasonic) DMF : 5 mg/mL (12.64 mM; Need ultrasonic) | ||
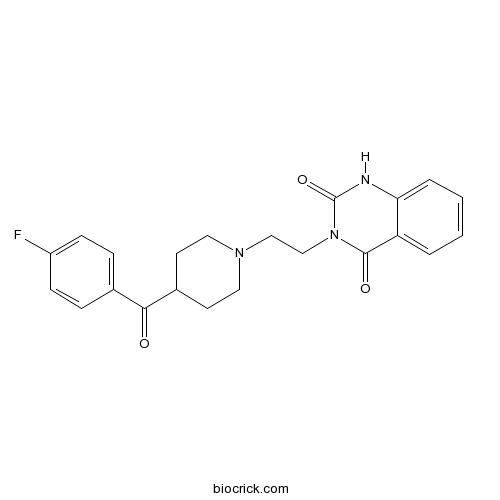
| Chemical Name | 3-[2-[4-(4-fluorobenzoyl)piperidin-1-yl]ethyl]-1H-quinazoline-2,4-dione | ||
| SMILES | C1CN(CCC1C(=O)C2=CC=C(C=C2)F)CCN3C(=O)C4=CC=CC=C4NC3=O | ||
| Standard InChIKey | FPCCSQOGAWCVBH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C22H22FN3O3/c23-17-7-5-15(6-8-17)20(27)16-9-11-25(12-10-16)13-14-26-21(28)18-3-1-2-4-19(18)24-22(26)29/h1-8,16H,9-14H2,(H,24,29) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ketanserin is a selective 5-HT receptor antagonist. Ketanserin also blocks hERG current (IhERG) in a concentration-dependent manner (IC50=0.11 μM).In Vitro:Ketanserin at 0.3 μM inhibits the voltage-dependent step current (IhERG.step) and tail current (IhERG.tail) of hERG channels with a 5-min exposure[1]. The synergistic effect observed for AA with 5-HT is, also, blocked by the 5-HT receptor blockers cyproheptadine (IC50=22.0±7 μM), Ketanserin (IC50=152±23 μM). Ketanserin (50-350 μM) inhibits the synergism by blocking the receptor in a dose-dependent manner. The IC50 value of Cyproheptadine is 22±7 μM and Ketanserin is 152±23 μM[2]. Ketanserin inhibits platelet aggregation with an IC50 of 240 (169-339) nM[3].In Vivo:Ketanserin is a 5-HT2A receptor antagonist. Ketanserin significantly reduces BDNF protein levels in numerous brain regions (CA1 and CA3 of the hippocampus, prefrontal cortex, central amygdaloid nucleus, dorsomedial hypothalamic nucleus, dentate gyrus, shell of the nucleus accumbens and midbrain periaqueductal gray). 5-HT2A antagonist Ketanserin can significantly reduce BDNF mRNA levels in various brain regions[4]. References: | |||||

Ketanserin Dilution Calculator

Ketanserin Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.5289 mL | 12.6445 mL | 25.2889 mL | 50.5779 mL | 63.2223 mL |
| 5 mM | 0.5058 mL | 2.5289 mL | 5.0578 mL | 10.1156 mL | 12.6445 mL |
| 10 mM | 0.2529 mL | 1.2644 mL | 2.5289 mL | 5.0578 mL | 6.3222 mL |
| 50 mM | 0.0506 mL | 0.2529 mL | 0.5058 mL | 1.0116 mL | 1.2644 mL |
| 100 mM | 0.0253 mL | 0.1264 mL | 0.2529 mL | 0.5058 mL | 0.6322 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Selective 5-HT2A serotonin receptor antagonist; can also be used to discriminate between 5-HT1D and 5-HT1B receptor subtypes.
- Lancifolin C
Catalog No.:BCN2019
CAS No.:74048-71-8
- Enoxacin (Penetrex)
Catalog No.:BCC3775
CAS No.:74011-58-8
- p-Hydroxy-cinnamic acid
Catalog No.:BCN5027
CAS No.:7400-08-0
- Mosloflavone
Catalog No.:BCN6796
CAS No.:740-33-0
- L-Arginine
Catalog No.:BCN2691
CAS No.:74-79-3
- Ethambutol
Catalog No.:BCC5195
CAS No.:74-55-5
- Kamebakaurin
Catalog No.:BCN8040
CAS No.:73981-34-7
- Cilostazol
Catalog No.:BCC2291
CAS No.:73963-72-1
- 7,8-Dimethoxy-1,3-dihydro-2H-3-benzazepin-2-one
Catalog No.:BCC8774
CAS No.:73942-87-7
- Isolimonexic acid
Catalog No.:BCN7141
CAS No.:73904-93-5
- Hispidone
Catalog No.:BCN4293
CAS No.:73891-72-2
- H-His-OMe.2HCl
Catalog No.:BCC2956
CAS No.:7389-87-9
- Macamide B
Catalog No.:BCN1366
CAS No.:74058-71-2
- Cudratricusxanthone A
Catalog No.:BCN7649
CAS No.:740810-42-8
- ACV 1
Catalog No.:BCC5989
CAS No.:740980-24-9
- Ketorolac
Catalog No.:BCC5190
CAS No.:74103-06-3
- Ketorolac tromethamine salt
Catalog No.:BCC4431
CAS No.:74103-07-4
- SKF 83822 hydrobromide
Catalog No.:BCC7252
CAS No.:74115-10-9
- Norjuziphine
Catalog No.:BCN3367
CAS No.:74119-87-2
- DSC
Catalog No.:BCC2800
CAS No.:74124-79-1
- Bisdethiobis(methylthio)gliotoxin
Catalog No.:BCN7351
CAS No.:74149-38-5
- Pimobendan
Catalog No.:BCC2294
CAS No.:74150-27-9
- 2,3-Dehydrokievitone
Catalog No.:BCN4294
CAS No.:74161-25-4
- R547
Catalog No.:BCC3927
CAS No.:741713-40-6
Ketanserin and Naftopidil Enhance the Potentiating Effect of Alpha-Methyl-Serotonin on the Neurally-Induced Contraction of Human Isolated Urinary Bladder Muscle Strips.[Pubmed:28361518]
Int Neurourol J. 2017 Mar 24;21(1):20-28.
PURPOSE: The aim of this study was to assess the potential involvement of a specific subtype of 5-hydroxytryptamine (5-HT), 5HT2 receptors in neurally-induced contractions of the human detrusor. METHODS: Contractile responses to electrical field stimulation (EFS) were examined in human isolated urinary bladder muscle strips. The potentiation of EFS-induced detrusor contraction was examined by adding cumulative concentrations of a 5-HT and 5-HT2 receptor agonist, alpha-methyl-serotonin (alpha-Me-5-HT) (1nM-100muM) in the presence or absence of a 5-HT2 antagonist, Ketanserin (5-HT2A>5-HT2C) or naftopidil (5-HT2B>5-HT2A) (0.3-3muM). RESULTS: 5-HT and alpha-Me-5-HT potentiated EFS-induced contraction with a maximal effect (Emax) of 37.6% and 38.6%, respectively, and with pEC50 (negative logarithm of the concentration required for a half-maximal response to an agonist) values of 8.3 and 6.8, respectively. Neither Ketanserin nor naftopidil at any concentration produced a rightward displacement of the alpha-Me-5-HT concentration response curve. Instead, the Emax of alpha-Me-5-HT increased in the presence of Ketanserin at 0.3-1muM and in the presence of naftopidil at 1muM to 51% and 56%, respectively, while the Emax in the presence of vehicle alone was 36%. The highest concentration (3muM) of either drug, however, fully reversed the enhancement. CONCLUSIONS: The potentiating effect of alpha-Me-5-HT on neurally-induced contraction of human urinary bladder muscle strips was not found to be mediated via any 5-HT2 receptor subtypes. The underlying mechanism for the enhancement of the alpha-Me-5-HT potentiating effect on detrusor contractility by Ketanserin and naftopidil remains unknown; however, our results suggest that these drugs may be useful for treating contractile dysfunction of the detrusor, as manifested in conditions such as underactive bladder.
Naltrexone but Not Ketanserin Antagonizes the Subjective, Cardiovascular, and Neuroendocrine Effects of Salvinorin-A in Humans.[Pubmed:26874330]
Int J Neuropsychopharmacol. 2016 Jul 5;19(7). pii: pyw016.
BACKGROUND: Salvinorin-A is a terpene found in the leaves of the plant Salvia divinorum. When administered to humans, salvinorin-A induces an intense but short-lasting modified state of awareness, sharing features with those induced by the classical serotonin-2A receptor agonist psychedelics. However, unlike substances such as psilocybin or mescaline, salvinorin-A shows agonist activity at the kappa-opioid receptor rather than at the serotonin-2A receptor. Here, we assessed the involvement of kappa-opioid receptor and serotonin-2A agonism in the subjective, cardiovascular, and neuroendocrine effects of salvinorin-A in humans. METHODS: We conducted a placebo-controlled, randomized, double-blind study with 2 groups of 12 healthy volunteers with experience with psychedelic drugs. There were 4 experimental sessions. In group 1, participants received the following treatment combinations: placebo+placebo, placebo+salvinorin-A, naltrexone+placebo, and naltrexone+salvinorin-A. Naltrexone, a nonspecific opioid receptor antagonist, was administered at a dose of 50mg orally. In group 2, participants received the treatment combinations: placebo+placebo, placebo+salvinorin-A, Ketanserin+placebo, and Ketanserin+salvinorin-A. Ketanserin, a selective serotonin-2A antagonist, was administered at a dose of 40mg orally. RESULTS: Inhalation of 1mg of vaporized salvinorin-A led to maximum plasma concentrations at 1 and 2 minutes after dosing. When administered alone, salvinorin-A severely reduced external sensory perception and induced intense visual and auditory modifications, increased systolic blood pressure, and cortisol and prolactin release. These effects were effectively blocked by naltrexone, but not by Ketanserin. CONCLUSIONS: Results support kappa opioid receptor agonism as the mechanism of action underlying the subjective and physiological effects of salvinorin-A in humans and rule out the involvement of a serotonin-2A-mediated mechanism.
Effects of ketanserin on experimental colitis in mice and macrophage function.[Pubmed:26865503]
Int J Mol Med. 2016 Mar;37(3):659-68.
Ketanserin is a selective 5-hydroxytryptamine (serotonin)-2A receptor (5-HT2AR) antagonist. Studies have suggested that Ketanserin exerts anti-inflammatory effects independent of the baroreflex; however, the mechanisms involved remain unclear. Thus, in the present study, we aimed to evaluate the effects of Ketanserin in colitis and the possible underlying mechanisms. The expression of 5-HT2AR was assessed in the colon tissues of patients with inflammatory bowel disease (IBD) and in mice with dextran sodium sulfate (DSS)-induced colitis. The therapeutic potential of Ketanserin was investigated in the mice with colitis. In the colon tissue samples from the patients with IBD, a high expression level of 5-HT2AR was observed. Treatment with Ketanserin attenuated the progression of experimental colitis in the mice, as indicated by body weight assessment, colon length, histological scores and cytokine release. The colonic macrophages from the Ketanserin-treated mice with colitis exhibited a decreased production of inflammatory cytokines, with M2 polarization and impaired migration. The knockdown of 5-HT2AR using siRNA partly abolished the inhibitory effects of Ketanserin on the release of pro-inflammatory cytokines in bone marrow derived-macrophages (BMDMs), thus demonstrating that the inhibitory effects of Ketanserin on the production of inflammatory cytokines are partly dependent on 5-HT2AR. Ketanserin also inhibited the activation of nuclear factor-kappaB (NF-kappaB) in BMDMs. In conclusion, the findings of the present study demonstrate that Ketanserin alleviates colitis. Its anti-inflammatory effects may be due to the promotion of the anti-inflammatory function of macrophages through 5-HT2AR/NF-kappaB.
Effects of ketanserin on microcirculatory alterations in septic shock: An open-label pilot study.[Pubmed:26264259]
J Crit Care. 2015 Dec;30(6):1156-62.
INTRODUCTION: Microcirculatory alterations in sepsis are associated with increased morbidity and mortality. These alterations occur despite macrohemodynamic resuscitation. Alternative pro-microcirculatory strategies, including vasodilatory drugs, have been suggested to improve capillary blood flow. Ketanserin, a serotonin receptor antagonist, is an attractive candidate because of its vasodilatory, antithrombotic, and anti-inflammatory effects. METHODS: This is an open-label pilot study on the effect of Ketanserin administration on microcirculatory alterations in septic shock, defined as microvascular flow index (MFI)2.9, the primary end point, was reached. RESULTS: Ten patients (Acute Physiology and Chronic Health Evaluation IV scores of 115 [100-136]) were included. Baseline MFI was 1.71 (1.31-2.32) and was significantly increasing to 2.96 (2.54-3.00; P=.021) during the Ketanserin infusion. The total Ketanserin dose was 0.09 (0.08-0.13) mg/kg per patient in 60 (30-60) minutes. In 3 patients (30%), the Ketanserin infusion was discontinued due to refractory hypotension. CONCLUSION: An improvement in microcirculatory perfusion was observed during Ketanserin administration in patients with septic shock after macrohemodynamic resuscitation. This finding needs further exploration in a placebo-controlled setting.


