SA 57Potent FAAH inhibitor CAS# 1346169-63-8 |
- Laminin (925-933)
Catalog No.:BCC1015
CAS No.:110590-60-8
- Epidermal Growth Factor Receptor Peptide (985-996)
Catalog No.:BCC1014
CAS No.:96249-43-3
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1346169-63-8 | SDF | Download SDF |
| PubChem ID | 44589122 | Appearance | Powder |
| Formula | C17H23ClN2O3 | M.Wt | 338.83 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 25 mM in DMSO and to 20 mM in ethanol | ||
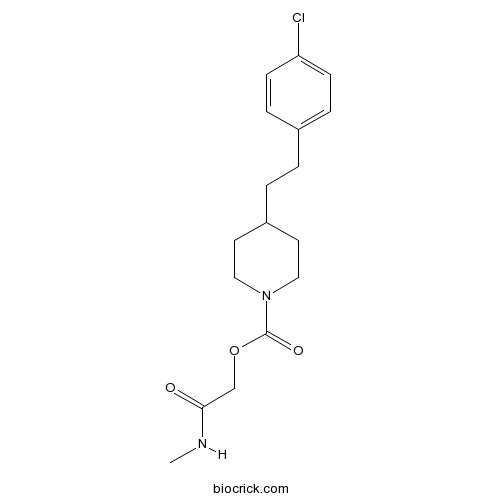
| Chemical Name | [2-(methylamino)-2-oxoethyl] 4-[2-(4-chlorophenyl)ethyl]piperidine-1-carboxylate | ||
| SMILES | CNC(=O)COC(=O)N1CCC(CC1)CCC2=CC=C(C=C2)Cl | ||
| Standard InChIKey | JFSSVCSHPDLFCM-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H23ClN2O3/c1-19-16(21)12-23-17(22)20-10-8-14(9-11-20)3-2-13-4-6-15(18)7-5-13/h4-7,14H,2-3,8-12H2,1H3,(H,19,21) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent inhibitor of fatty acid amide hydrolase (FAAH) (IC50 <10 nM). Also inhibits MAGL at higher concentrations (IC50 values are 410 nM and 1.4 μM respectively). Inhibits both human and mouse FAAH enzymes. Exhibits inhibitory activity against FAAH, MAGL and ABHD6 in vivo. |

SA 57 Dilution Calculator

SA 57 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.9513 mL | 14.7567 mL | 29.5133 mL | 59.0267 mL | 73.7833 mL |
| 5 mM | 0.5903 mL | 2.9513 mL | 5.9027 mL | 11.8053 mL | 14.7567 mL |
| 10 mM | 0.2951 mL | 1.4757 mL | 2.9513 mL | 5.9027 mL | 7.3783 mL |
| 50 mM | 0.059 mL | 0.2951 mL | 0.5903 mL | 1.1805 mL | 1.4757 mL |
| 100 mM | 0.0295 mL | 0.1476 mL | 0.2951 mL | 0.5903 mL | 0.7378 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Planchol E
Catalog No.:BCN6882
CAS No.:1346137-02-7
- LY2795050
Catalog No.:BCC1719
CAS No.:1346133-08-1
- ML 154
Catalog No.:BCC8022
CAS No.:1345964-89-7
- 5,7-Di-O-methylquercetin
Catalog No.:BCN3386
CAS No.:13459-07-9
- CYM 50308
Catalog No.:BCC6260
CAS No.:1345858-76-5
- Altiratinib
Catalog No.:BCC6385
CAS No.:1345847-93-9
- Arginase inhibitor 1
Catalog No.:BCC4034
CAS No.:1345808-25-4
- ETP-46464
Catalog No.:BCC3913
CAS No.:1345675-02-6
- AM095
Catalog No.:BCC1351
CAS No.:1345614-59-6
- Atorvastatin Calcium
Catalog No.:BCC2319
CAS No.:134523-03-8
- U 90042
Catalog No.:BCC7465
CAS No.:134516-99-7
- Gardenoin J
Catalog No.:BCN7666
CAS No.:1345109-46-7
- ML 218 hydrochloride
Catalog No.:BCC6207
CAS No.:1346233-68-8
- Stavudine sodium
Catalog No.:BCC4263
CAS No.:134624-73-0
- Eriocitrin
Catalog No.:BCN1208
CAS No.:13463-28-0
- Zinc Pyrithione
Catalog No.:BCC5008
CAS No.:13463-41-7
- ML 240
Catalog No.:BCC5604
CAS No.:1346527-98-7
- GSK503
Catalog No.:BCC6386
CAS No.:1346572-63-1
- GSK126
Catalog No.:BCC1604
CAS No.:1346574-57-9
- GSK621
Catalog No.:BCC6517
CAS No.:1346607-05-3
- GSK343
Catalog No.:BCC1607
CAS No.:1346704-33-3
- Lamivudine
Catalog No.:BCC3801
CAS No.:134678-17-4
- Linderane
Catalog No.:BCN5023
CAS No.:13476-25-0
- A 412997 dihydrochloride
Catalog No.:BCC6224
CAS No.:1347744-96-0
The endocannabinoid hydrolysis inhibitor SA-57: Intrinsic antinociceptive effects, augmented morphine-induced antinociception, and attenuated heroin seeking behavior in mice.[Pubmed:27890602]
Neuropharmacology. 2017 Mar 1;114:156-167.
Although opioids are highly efficacious analgesics, their abuse potential and other untoward side effects diminish their therapeutic utility. The addition of non-opioid analgesics offers a promising strategy to reduce required antinociceptive opioid doses that concomitantly reduce opioid-related side effects. Inhibitors of the primary endocannabinoid catabolic enzymes fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL) show opioid-sparing effects in preclinical models of pain. As simultaneous inhibition of these enzymes elicits enhanced antinociceptive effects compared with single enzyme inhibition, the present study tested whether the dual FAAH-MAGL inhibitor SA-57 [4-[2-(4-chlorophenyl)ethyl]-1-piperidinecarboxylic acid 2-(methylamino)-2-oxoethyl ester] produces morphine-sparing antinociceptive effects, without major side effects associated with either drug class. SA-57 dose-dependently reversed mechanical allodynia in the constriction injury (CCI) of the sciatic nerve model of neuropathic pain and carrageenan inflammatory pain model. As previously reported, SA-57 was considerably more potent in elevating anandamide (AEA) than 2-arachidonyl glycerol (2-AG) in brain. Its anti-allodynic effects required cannabinoid (CB)1 and CB2 receptors; however, only CB2 receptors were necessary for the anti-edematous effects in the carrageenan assay. Although high doses of SA-57 alone were required to produce antinociception, low doses of this compound, which elevated AEA and did not affect 2-AG brain levels, augmented the antinociceptive effects of morphine, but lacked cannabimimetic side effects. Because of the high abuse liability of opioids and implication of the endocannabinoid system in the reinforcing effects of opioids, the final experiment tested whether SA-57 would alter heroin seeking behavior. Strikingly, SA-57 reduced heroin-reinforced nose poke behavior and the progressive ratio break point for heroin. In conclusion, the results of the present study suggest that inhibition of endocannabinoid degradative enzymes represents a promising therapeutic approach to decrease effective doses of opioids needed for clinical pain control, and may also possess therapeutic potential to reduce opioid abuse.
Discriminative Stimulus Properties of the Endocannabinoid Catabolic Enzyme Inhibitor SA-57 in Mice.[Pubmed:27307500]
J Pharmacol Exp Ther. 2016 Aug;358(2):306-14.
Whereas the inhibition of fatty acid amide hydrolase (FAAH) or monoacylglycerol lipase (MAGL), the respective major hydrolytic enzymes of N-arachidonoyl ethanolamine (AEA) and 2-arachidonoylglycerol (2-AG), elicits no or partial substitution for Delta(9)-tetrahydrocannabinol (THC) in drug-discrimination procedures, combined inhibition of both enzymes fully substitutes for THC, as well as produces a constellation of cannabimimetic effects. The present study tested whether C57BL/6J mice would learn to discriminate the dual FAAH-MAGL inhibitor SA-57 (4-[2-(4-chlorophenyl)ethyl]-1-piperidinecarboxylic acid 2-(methylamino)-2-oxoethyl ester) from vehicle in the drug-discrimination paradigm. In initial experiments, 10 mg/kg SA-57 fully substituted for CP55,940 ((-)-cis-3-[2-hydroxy-4-(1,1-dimethylheptyl)phenyl]-trans-4-(3-hydroxypropyl)cycl ohexanol), a high-efficacy CB1 receptor agonist in C57BL/6J mice and for AEA in FAAH (-/-) mice. Most (i.e., 23 of 24) subjects achieved criteria for discriminating SA-57 (10 mg/kg) from vehicle within 40 sessions, with full generalization occurring 1 to 2 hours postinjection. CP55,940, the dual FAAH-MAGL inhibitor JZL195 (4-nitrophenyl 4-(3-phenoxybenzyl)piperazine-1-carboxylate), and the MAGL inhibitors MJN110 (2,5-dioxopyrrolidin-1-yl 4-(bis(4-chlorophenyl)methyl)piperazine-1-carboxylate) and JZL184 (4-[Bis(1,3-benzodioxol-5-yl)hydroxymethyl]-1-piperidinecarboxylic acid 4-nitrophenyl ester) fully substituted for SA-57. Although the FAAH inhibitors PF-3845 ((N-3-pyridinyl-4-[[3-[[5-(trifluoromethyl)-2-pyridinyl]oxy]phenyl]methyl]-1-pipe ridinecarboxamide) and URB597 (cyclohexylcarbamic acid 3'-(aminocarbonyl)-[1,1'-biphenyl]-3-yl ester) did not substitute for SA-57, PF-3845 produced a 2-fold leftward shift in the MJN110 substitution dose-response curve. In addition, the CB1 receptor antagonist rimonabant blocked the generalization of SA-57, as well as substitution of CP55,940, JZL195, MJN110, and JZL184. These findings suggest that MAGL inhibition plays a major role in the CB1 receptor-mediated SA-57 training dose, which is further augmented by FAAH inhibition.


