SyringaresinolCAS# 487-35-4 |
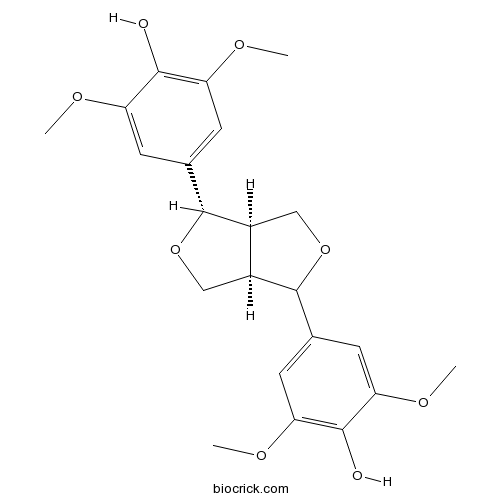
- DL-Syringaresinol
Catalog No.:BCN6053
CAS No.:1177-14-6
- (+)-Syringaresinol
Catalog No.:BCN7496
CAS No.:21453-69-0
- Episyringaresinol
Catalog No.:BCN7023
CAS No.:51152-20-6
- (-)-Syringaresinol
Catalog No.:BCN3417
CAS No.:6216-81-5
- (-)-Episyringaresinol
Catalog No.:BCN9229
CAS No.:6216-82-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 487-35-4 | SDF | Download SDF |
| PubChem ID | 332426 | Appearance | Powder |
| Formula | C22H26O8 | M.Wt | 418.4 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
| Chemical Name | 4-[(3aR,6S,6aR)-6-(4-hydroxy-3,5-dimethoxyphenyl)-1,3,3a,4,6,6a-hexahydrofuro[3,4-c]furan-3-yl]-2,6-dimethoxyphenol | ||
| SMILES | COC1=CC(=CC(=C1O)OC)C2C3COC(C3CO2)C4=CC(=C(C(=C4)OC)O)OC | ||
| Standard InChIKey | KOWMJRJXZMEZLD-VGBAVMOLSA-N | ||
| Standard InChI | InChI=1S/C22H26O8/c1-25-15-5-11(6-16(26-2)19(15)23)21-13-9-30-22(14(13)10-29-21)12-7-17(27-3)20(24)18(8-12)28-4/h5-8,13-14,21-24H,9-10H2,1-4H3/t13-,14-,21+,22?/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | (-)-Syringaresinol may be a potential chemotherapeutic agent for the treatment of cancer; it against H/R-induced cardiomyocyte injury and death, the degradation of HIF-1α through activation of FOXO3 is a potential therapeutic strategy for ischemia-related diseases. Syringaresinol induces vasorelaxation by enhancing NO production in endothelial cells via two distinct mechanisms, phosphatidylinositol 3-kinase/Akt- and PLC/Ca(2+)/CaMKKβ-dependent eNOS phosphorylation and Ca(2+)-dependent eNOS dimerization. |
| Targets | HIF | mTOR | ROS | NO | NOS | Akt | AMPK | Calcium Channel | PI3K | CDK | p21 | Bcl-2/Bax | PARP | Caspase |
| In vitro | Syringaresinol protects against hypoxia/reoxygenation-induced cardiomyocytes injury and death by destabilization of HIF-1α in a FOXO3-dependent mechanism.[Pubmed: 25415049]Oncotarget. 2015 Jan 1;6(1):43-55.Hypoxia-inducible factor 1 (HIF-1) is a master regulator of hypoxic response and has been a prime therapeutic target for ischemia/reperfusion (I/R)-derived myocardial dysfunction and tissue damage. There is also increasing evidence that HIF-1 plays a central role in regulating aging, both through interactions with key longevity factors including Sirtuins and mTOR, as well as by directly promoting longevity in Caenorhabditis elegans. (-)-Syringaresinol inhibits proliferation of human promyelocytic HL-60 leukemia cells via G1 arrest and apoptosis.[Pubmed: 18486907]Int Immunopharmacol. 2008 Jul;8(7):967-73.
|
| Kinase Assay | Syringaresinol causes vasorelaxation by elevating nitric oxide production through the phosphorylation and dimerization of endothelial nitric oxide synthase.[Pubmed: 22170035]Exp Mol Med. 2012 Mar 31;44(3):191-201.Nitric oxide (NO) produced by endothelial NO synthase (eNOS) plays an important role in vascular functions, including vasorelaxation. |
| Structure Identification | Bioorg Med Chem Lett. 2015 Jan 15;25(2):307-9.Enantioselective induction of SIRT1 gene by syringaresinol from Panax ginseng berry and Acanthopanax senticosus Harms stem.[Pubmed: 25479772]
|

Syringaresinol Dilution Calculator

Syringaresinol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.3901 mL | 11.9503 mL | 23.9006 mL | 47.8011 mL | 59.7514 mL |
| 5 mM | 0.478 mL | 2.3901 mL | 4.7801 mL | 9.5602 mL | 11.9503 mL |
| 10 mM | 0.239 mL | 1.195 mL | 2.3901 mL | 4.7801 mL | 5.9751 mL |
| 50 mM | 0.0478 mL | 0.239 mL | 0.478 mL | 0.956 mL | 1.195 mL |
| 100 mM | 0.0239 mL | 0.1195 mL | 0.239 mL | 0.478 mL | 0.5975 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Scopoline
Catalog No.:BCN1942
CAS No.:487-27-4
- Elemicin
Catalog No.:BCN2818
CAS No.:487-11-6
- Citropten
Catalog No.:BCN4831
CAS No.:487-06-9
- Cyclocalopin A
Catalog No.:BCN5587
CAS No.:486430-94-8
- O-Acetylcyclocalopin A
Catalog No.:BCN5586
CAS No.:486430-93-7
- CIQ
Catalog No.:BCC7862
CAS No.:486427-17-2
- AZD2858
Catalog No.:BCC4509
CAS No.:486424-20-8
- Thermopsine
Catalog No.:BCN2603
CAS No.:486-90-8
- Anagyrine
Catalog No.:BCN3049
CAS No.:486-89-5
- alpha-Isolupanine
Catalog No.:BCN7989
CAS No.:486-87-3
- N-Methylcytisine
Catalog No.:BCN1266
CAS No.:486-86-2
- Harman
Catalog No.:BCN3998
CAS No.:486-84-0
- Pinoresinol
Catalog No.:BCN5591
CAS No.:487-36-5
- Phillygenin
Catalog No.:BCN2653
CAS No.:487-39-8
- Phillyrin
Catalog No.:BCN1096
CAS No.:487-41-2
- Ononetin
Catalog No.:BCC6367
CAS No.:487-49-0
- Butein
Catalog No.:BCN5592
CAS No.:487-52-5
- Hypaphorine
Catalog No.:BCN2775
CAS No.:487-58-1
- Kainic acid
Catalog No.:BCC6572
CAS No.:487-79-6
- Lindelofine
Catalog No.:BCN2043
CAS No.:487-99-0
- HI TOPK 032
Catalog No.:BCC6225
CAS No.:487020-03-1
- AR-A014418
Catalog No.:BCC1366
CAS No.:487021-52-3
- SNAP 94847 hydrochloride
Catalog No.:BCC7658
CAS No.:487051-12-7
- Curcumol
Catalog No.:BCN5976
CAS No.:4871-97-0
Enantioselective induction of SIRT1 gene by syringaresinol from Panax ginseng berry and Acanthopanax senticosus Harms stem.[Pubmed:25479772]
Bioorg Med Chem Lett. 2015 Jan 15;25(2):307-9.
Syringaresinol exists either exclusively as one enantiomer or enantiomeric mixtures in plant foods. We found that (+)-Syringaresinol, but not (-)-Syringaresinol, upregulates silent information regulator two ortholog 1 (SIRT1) gene expression, and thus, Panax ginseng berry with predominantly high contents of (+)-Syringaresinol exhibits higher activity in inducing SIRT1 gene expression than Acanthopanax senticosus Harms stem with almost equal proportion of the two enantiomers. These findings highlight the importance of the absolute configuration of Syringaresinol for the biological activity.
Syringaresinol protects against hypoxia/reoxygenation-induced cardiomyocytes injury and death by destabilization of HIF-1alpha in a FOXO3-dependent mechanism.[Pubmed:25415049]
Oncotarget. 2015 Jan 1;6(1):43-55.
Hypoxia-inducible factor 1 (HIF-1) is a master regulator of hypoxic response and has been a prime therapeutic target for ischemia/reperfusion (I/R)-derived myocardial dysfunction and tissue damage. There is also increasing evidence that HIF-1 plays a central role in regulating aging, both through interactions with key longevity factors including Sirtuins and mTOR, as well as by directly promoting longevity in Caenorhabditis elegans.We investigated a novel function and the underlying mechanism of Syringaresinol, a lignan compound, in modulation of HIF-1 and protection against cellular damage and death in a cardiomyocyte model of I/R injury. Syringaresinol caused destabilization of HIF-1alpha following H/R and then protected against hypoxia/reoxygenation (H/R)-induced cellular damage, apoptosis, and mitochondrial dysfunction in a dose-dependent manner. Knock-down of FOXO3 by specific siRNAs completely abolished the ability of Syringaresinol to inhibit HIF-1 stabilization and apoptosis caused by H/R. Syringaresinol stimulated the nuclear localization and activity of FOXO3 leading to increased expression of antioxidant genes and decreased levels of reactive oxygen species (ROS) following H/R. Our results provide a new mechanistic insight into a functional role of Syringaresinol against H/R-induced cardiomyocyte injury and death. The degradation of HIF-1alpha through activation of FOXO3 is a potential therapeutic strategy for ischemia-related diseases.
Syringaresinol causes vasorelaxation by elevating nitric oxide production through the phosphorylation and dimerization of endothelial nitric oxide synthase.[Pubmed:22170035]
Exp Mol Med. 2012 Mar 31;44(3):191-201.
Nitric oxide (NO) produced by endothelial NO synthase (eNOS) plays an important role in vascular functions, including vasorelaxation. We here investigated the pharmacological effect of the natural product Syringaresinol on vascular relaxation and eNOS-mediated NO production as well as its underlying biochemical mechanism in endothelial cells. Treatment of aortic rings from wild type, but not eNOS(-/-) mice, with Syringaresinol induced endothelium-dependent relaxation, which was abolished by addition of the NOS inhibitor N(G)-monomethyl-L-arginine. Treatment of human endothelial cells and mouse aortic rings with Syringaresinol increased NO production, which was correlated with eNOS phosphorylation via the activation of Akt and AMP kinase (AMPK) as well as elevation of intracellular Ca(2+) levels. A phospholipase C (PLC) inhibitor blocked the increases in intracellular Ca(2+) levels, AMPK-dependent eNOS phosphorylation, and NO production, but not Akt activation, in Syringaresinol- treated endothelial cells. Syringaresinol-induced AMPK activation was inhibited by co-treatment with PLC inhibitor, Ca(2+) chelator, calmodulin antagonist, and CaMKKbeta siRNA. This compound also increased eNOS dimerization, which was inhibited by a PLC inhibitor and a Ca(2+)-chelator. The chemicals that inhibit eNOS phosphorylation and dimerization attenuated vasorelaxation and cGMP production. These results suggest that Syringaresinol induces vasorelaxation by enhancing NO production in endothelial cells via two distinct mechanisms, phosphatidylinositol 3-kinase/Akt- and PLC/Ca(2+)/CaMKKbeta-dependent eNOS phosphorylation and Ca(2+)-dependent eNOS dimerization.
(-)-Syringaresinol inhibits proliferation of human promyelocytic HL-60 leukemia cells via G1 arrest and apoptosis.[Pubmed:18486907]
Int Immunopharmacol. 2008 Jul;8(7):967-73.
We examined the effect of (-)-Syringaresinol, a furofuran-type lignan isolated from Daphne genkwa, on cell cycle regulation in HL-60 human promyelocytic leukemia cells in vitro. (-)-Syringaresinol decreased the viability of HL-60 cells by inducing G(1) arrest followed by apoptosis in a dose- and time-dependent manner. The G(0)/G(1) phase of the cell cycle is regulated by cyclin-dependent kinases (Cdk), cyclins and cyclin-dependent kinase inhibitors (Cdki). We show by western blot analysis, that the (-)-Syringaresinol-induced G(1) arrest was mediated through the increased expression of Cdki proteins (p21(cip1/waf1) and p27(kip1)) with a simultaneous decrease in cdk2, cdk4, cdk6, cyclin D(1), cyclin D(2), and cyclin E expression. The induction of apoptosis after treatment with (-)-Syringaresinol for 24 h was demonstrated by morphological changes, DNA fragmentation, altered ratio of Bax/Bcl-2, cleavage of poly(ADP-ribose) polymerase and flow cytometry analysis. (-)-Syringaresinol also induced cytochrome c release and activation of caspase-3 and caspase-9. To our knowledge, this is the first time that (-)-Syringaresinol has been reported to potently inhibit the proliferation of human promyelocytic HL-60 cells through G(1) arrest and induction of apoptosis. These findings suggest that (-)-Syringaresinol may be a potential chemotherapeutic agent for the treatment of cancer.


