SNAP 94847 hydrochloridePotent and selective MCH1 antagonist CAS# 487051-12-7 |
- 3,3'-Diindolylmethane
Catalog No.:BCC1306
CAS No.:1968-05-4
- BAM7
Catalog No.:BCC1397
CAS No.:331244-89-4
- Bendamustine HCl
Catalog No.:BCC1153
CAS No.:3543-75-7
- Betulinic acid
Catalog No.:BCN5524
CAS No.:472-15-1
- Brassinolide
Catalog No.:BCC1438
CAS No.:72962-43-7
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 487051-12-7 | SDF | Download SDF |
| PubChem ID | 56972235 | Appearance | Powder |
| Formula | C29H33ClF2N2O2 | M.Wt | 515.03 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 100 mM in ethanol | ||
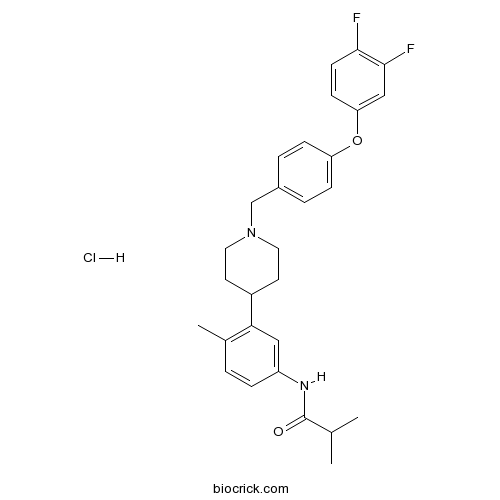
| Chemical Name | N-[3-[1-[[4-(3,4-difluorophenoxy)phenyl]methyl]piperidin-4-yl]-4-methylphenyl]-2-methylpropanamide;hydrochloride | ||
| SMILES | CC1=C(C=C(C=C1)NC(=O)C(C)C)C2CCN(CC2)CC3=CC=C(C=C3)OC4=CC(=C(C=C4)F)F.Cl | ||
| Standard InChIKey | DEDUDFNRQKUBRH-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C29H32F2N2O2.ClH/c1-19(2)29(34)32-23-7-4-20(3)26(16-23)22-12-14-33(15-13-22)18-21-5-8-24(9-6-21)35-25-10-11-27(30)28(31)17-25;/h4-11,16-17,19,22H,12-15,18H2,1-3H3,(H,32,34);1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent melanin-concentrating hormone receptor 1 (MCH1) antagonist (Ki = 2.2 nM, KD = 530 pM) that displays > 80-fold and > 500-fold selectivity over α1A and D2 receptors respectively. Increases neurogenesis in the dentate gyrus and decreases food-reinforced operant responding in vivo. Exhibits anxiolytic activity and is orally active. |

SNAP 94847 hydrochloride Dilution Calculator

SNAP 94847 hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 1.9416 mL | 9.7082 mL | 19.4163 mL | 38.8327 mL | 48.5409 mL |
| 5 mM | 0.3883 mL | 1.9416 mL | 3.8833 mL | 7.7665 mL | 9.7082 mL |
| 10 mM | 0.1942 mL | 0.9708 mL | 1.9416 mL | 3.8833 mL | 4.8541 mL |
| 50 mM | 0.0388 mL | 0.1942 mL | 0.3883 mL | 0.7767 mL | 0.9708 mL |
| 100 mM | 0.0194 mL | 0.0971 mL | 0.1942 mL | 0.3883 mL | 0.4854 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- AR-A014418
Catalog No.:BCC1366
CAS No.:487021-52-3
- HI TOPK 032
Catalog No.:BCC6225
CAS No.:487020-03-1
- Lindelofine
Catalog No.:BCN2043
CAS No.:487-99-0
- Kainic acid
Catalog No.:BCC6572
CAS No.:487-79-6
- Hypaphorine
Catalog No.:BCN2775
CAS No.:487-58-1
- Butein
Catalog No.:BCN5592
CAS No.:487-52-5
- Ononetin
Catalog No.:BCC6367
CAS No.:487-49-0
- Phillyrin
Catalog No.:BCN1096
CAS No.:487-41-2
- Phillygenin
Catalog No.:BCN2653
CAS No.:487-39-8
- Pinoresinol
Catalog No.:BCN5591
CAS No.:487-36-5
- Syringaresinol
Catalog No.:BCN3042
CAS No.:487-35-4
- Scopoline
Catalog No.:BCN1942
CAS No.:487-27-4
- Curcumol
Catalog No.:BCN5976
CAS No.:4871-97-0
- Heleurine
Catalog No.:BCN1953
CAS No.:488-00-6
- Thesinine
Catalog No.:BCN1990
CAS No.:488-02-8
- 3-Methylcatechol
Catalog No.:BCN3925
CAS No.:488-17-5
- Allitol
Catalog No.:BCN5593
CAS No.:488-44-8
- vibo-Quercitol
Catalog No.:BCN5594
CAS No.:488-76-6
- D-Ribitol-5-phosphate
Catalog No.:BCC4838
CAS No.:35320-17-3
- D-arabinitol
Catalog No.:BCN5595
CAS No.:488-82-4
- Furan-3-carboxylic acid
Catalog No.:BCN6397
CAS No.:488-93-7
- N-Acetylstepharine
Catalog No.:BCN7566
CAS No.:4880-87-9
- Elesclomol (STA-4783)
Catalog No.:BCC2337
CAS No.:488832-69-5
- Icariin
Catalog No.:BCN6311
CAS No.:489-32-7
Effects of the MCH1 receptor antagonist SNAP 94847 on high-fat food-reinforced operant responding and reinstatement of food seeking in rats.[Pubmed:19340414]
Psychopharmacology (Berl). 2009 Jul;205(1):129-40.
RATIONALE AND OBJECTIVES: The melanin-concentrating hormone 1 (MCH1) receptors play an important role in home-cage food consumption in rodents, but their role in operant high-fat food-reinforced responding or reinstatement of food seeking in animal models is unknown. Here, we used the MCH1 receptor antagonist SNAP 94847 to explore these questions. MATERIALS AND METHODS: In experiment 1, we trained food-restricted rats (16 g/day of nutritionally balanced rodent diet) to lever press for high-fat (35%) pellets (3-h/day, every other day) for 14 sessions. We then tested the effect of SNAP 94847 (3-30 mg/kg, intraperitoneal (i.p.)) on food-reinforced operant responding. In experiments 2 and 3, we trained rats to lever press for the food pellets (9 to 14 3-h sessions) and subsequently extinguished the food-reinforced lever responding by removing the food (10 to 17 sessions). We then tested the effect of SNAP 94847 on reinstatement of food seeking induced by MCH (20 microg, intracerebroventricular), noncontingent delivery of three pellets during the first minute of the test session (pellet-priming), contingent tone-light cues previously associated with pellet delivery (cue), or the pharmacological stressor yohimbine (2 mg/kg, i.p.). RESULTS: Systemic injections of SNAP 94847 decreased food-reinforced operant responding and MCH-induced reinstatement of food seeking. SNAP 94847 had no effect on pellet-priming-, cue-, or yohimbine-induced reinstatement. CONCLUSIONS: Results indicate that MCH1 receptors are involved in food-reinforced operant responding but not in reinstatement induced by acute exposure to high-fat food, food cues, or the stress-like state induced by yohimbine. These results suggest that different mechanisms mediate food-reinforced operant responding and reinstatement of food seeking.
Synthesis and SAR investigations for novel melanin-concentrating hormone 1 receptor (MCH1) antagonists part 2: A hybrid strategy combining key fragments of HTS hits.[Pubmed:17668922]
J Med Chem. 2007 Aug 9;50(16):3883-90.
A novel series of melanin-concentrating hormone (MCH1) receptor antagonists based on combining key fragments from the high-throughput screening (HTS) hits compound 2 (SNAP 7941) and compound 5 (chlorohaloperidol) are described. The resultant analogs, exemplified by compounds 11a-11h, 15a-15h, and 16a-16g, were evaluated in in vitro and in vivo assays for their potential in treatment of mood disorders. From further SAR investigations, N-(3-{1-[4-(3,4-difluorophenoxy)benzyl]-4-piperidinyl}-4-methylphenyl)-2-methylpr opanamide (16g, SNAP 94847) was identified to be a high affinity and selective ligand for the MCH1 receptor. Compound 16g also shows good oral bioavailability (59%) and exhibits a brain/plasma ratio of 2.3 in rats. Compound 16g showed in vivo inhibition of a centrally induced MCH-induced drinking effect and exhibited a dose-dependent anxiolytic effect in the rat social interaction model.
Efficacy of the MCHR1 antagonist N-[3-(1-{[4-(3,4-difluorophenoxy)phenyl]methyl}(4-piperidyl))-4-methylphenyl]-2-m ethylpropanamide (SNAP 94847) in mouse models of anxiety and depression following acute and chronic administration is independent of hippocampal neurogenesis.[Pubmed:17237257]
J Pharmacol Exp Ther. 2007 Apr;321(1):237-48.
Melanin-concentrating hormone (MCH) is a hypothalamic neuropeptide that plays a role in the modulation of food intake and mood. In rodents, the actions of MCH are mediated via the MCHR1 receptor. The goal of this study was to investigate the effects of acute (1 h) and chronic (28 days) p.o. dosing of a novel MCHR1 antagonist, N-[3-(1-{[4-(3,4-difluorophenoxy)-phenyl]methyl}(4-piperidyl))-4-methylphenyl]-2- methylpropanamide (SNAP 94847), in three mouse models predictive of antidepressant/anxiolytic-like activity: novelty suppressed feeding (NSF) in 129S6/SvEvTac mice and light/dark paradigm (L/D) and forced swim test (FST) in BALB/cJ mice. A significant increase in the time spent in the light compartment of the L/D box was observed in response to acute and chronic treatment with SNAP 94847. An anxiolytic/antidepressant-like effect was found in the NSF test after acute and chronic treatment, whereas no effect was observed in the FST. Because neurogenesis in the dentate gyrus has been shown to be a requirement for the effects of antidepressants in the NSF test, we investigated whether neurogenesis was required for the effect of SNAP 94847. We showed that chronic treatment with SNAP 94847 stimulated proliferation of progenitors in the dentate gyrus. The efficacy of SNAP 94847 in the NSF test, however, was unaltered in mice in which neurogenesis was suppressed by X-irradiation. These results indicate that SNAP 94847 has a unique anxiolytic-like profile after both acute and chronic administration and that its mechanism of action is distinct from that of selective serotonin reuptake inhibitors and tricyclic antidepressants.


