Furan-3-carboxylic acidCAS# 488-93-7 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 488-93-7 | SDF | Download SDF |
| PubChem ID | 10268 | Appearance | Powder |
| Formula | C5H4O3 | M.Wt | 112.08 |
| Type of Compound | Phenols | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
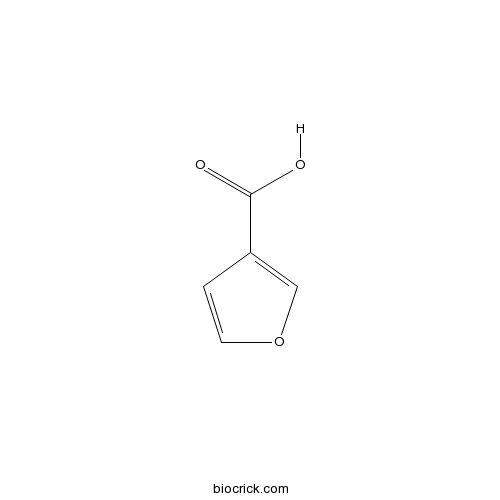
| Chemical Name | furan-3-carboxylic acid | ||
| SMILES | C1=COC=C1C(=O)O | ||
| Standard InChIKey | IHCCAYCGZOLTEU-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C5H4O3/c6-5(7)4-1-2-8-3-4/h1-3H,(H,6,7) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Furan-3-carboxylic acid is a natural product from Corynebacterium. |
| Structure Identification | Tetrahedron Letters, 2004, 45(29):5689-5691.Convenient synthesis of furan-3-carboxylic acid and derivatives[Reference: WebLink]A convenient synthesis of Furan-3-carboxylic acid and derivatives from aromatization of 4-trichloroacetyl-2,3-dihydrofuran followed by nucleophilic displacement of the trichloromethyl group by hydroxide, alcohols, and amines, is presented. |

Furan-3-carboxylic acid Dilution Calculator

Furan-3-carboxylic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 8.9222 mL | 44.611 mL | 89.222 mL | 178.444 mL | 223.055 mL |
| 5 mM | 1.7844 mL | 8.9222 mL | 17.8444 mL | 35.6888 mL | 44.611 mL |
| 10 mM | 0.8922 mL | 4.4611 mL | 8.9222 mL | 17.8444 mL | 22.3055 mL |
| 50 mM | 0.1784 mL | 0.8922 mL | 1.7844 mL | 3.5689 mL | 4.4611 mL |
| 100 mM | 0.0892 mL | 0.4461 mL | 0.8922 mL | 1.7844 mL | 2.2305 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- D-arabinitol
Catalog No.:BCN5595
CAS No.:488-82-4
- D-Ribitol-5-phosphate
Catalog No.:BCC4838
CAS No.:35320-17-3
- vibo-Quercitol
Catalog No.:BCN5594
CAS No.:488-76-6
- Allitol
Catalog No.:BCN5593
CAS No.:488-44-8
- 3-Methylcatechol
Catalog No.:BCN3925
CAS No.:488-17-5
- Thesinine
Catalog No.:BCN1990
CAS No.:488-02-8
- Heleurine
Catalog No.:BCN1953
CAS No.:488-00-6
- Curcumol
Catalog No.:BCN5976
CAS No.:4871-97-0
- SNAP 94847 hydrochloride
Catalog No.:BCC7658
CAS No.:487051-12-7
- AR-A014418
Catalog No.:BCC1366
CAS No.:487021-52-3
- HI TOPK 032
Catalog No.:BCC6225
CAS No.:487020-03-1
- Lindelofine
Catalog No.:BCN2043
CAS No.:487-99-0
- N-Acetylstepharine
Catalog No.:BCN7566
CAS No.:4880-87-9
- Elesclomol (STA-4783)
Catalog No.:BCC2337
CAS No.:488832-69-5
- Icariin
Catalog No.:BCN6311
CAS No.:489-32-7
- Limocitrin
Catalog No.:BCN3346
CAS No.:489-33-8
- Gossypetin
Catalog No.:BCN8075
CAS No.:489-35-0
- Globulol
Catalog No.:BCN6901
CAS No.:489-41-8
- Guaiazulen
Catalog No.:BCC8180
CAS No.:489-84-9
- Guaiol
Catalog No.:BCN6619
CAS No.:489-86-1
- ML 213
Catalog No.:BCC6213
CAS No.:489402-47-3
- SBC-115076
Catalog No.:BCC6440
CAS No.:489415-96-5
- a-Truxilline
Catalog No.:BCN1947
CAS No.:490-17-5
- Beta-Tocotrienol
Catalog No.:BCN3725
CAS No.:490-23-3
Antioxidant activity and inhibitory effects of 2-hydroxy-3-methylcyclopent-2-enone isolated from ribose-histidine Maillard reaction products on aldose reductase and tyrosinase.[Pubmed:29513344]
Food Funct. 2018 Mar 1;9(3):1790-1799.
This study aimed to better understand the functional properties of ribose and 20 amino acid Maillard reaction products (MRPs). The ABTS(+) radical scavenging ability of the ribose-20 amino acid MRPs was evaluated. Among the MRPs, ribose-histidine MRPs (RH-MRPs) showed the highest inhibitory activities on the ABTS(+) radical scavenging ability, aldose reductase (AR), and tyrosinase compared to other MRPs. Functional compounds with antioxidant and AR inhibitory activities have been recognized as an important strategy in the prevention and treatment of diabetic complications, and the search for tyrosinase inhibitors is important for the treatment of hyperpigmentation, development of skin-whitening agents, and use as preservatives in the food industry. On this basis, we sought to isolate and identify compounds with inhibitory activities against AR and tyrosinase. RH-MRPs were heated at 120 degrees C for 2 h and fractionated using four solvents: methylene chloride (MC), ethyl acetate, n-butanol, and water. The highest inhibitions were found in the MC fraction. The two compounds from this fraction were purified by silica gel column and preparative thin layer chromatography, and identified as 2-hydroxy-3-methylcyclopent-2-enone and Furan-3-carboxylic acid. AR inhibition, tyrosinase inhibition, and ABTS(+) scavenging (IC50) of 2-hydroxy-3-methylcyclopent-2-enone were 4.47, 721.91 and 9.81 mug mL(-1), respectively. In this study, inhibitory effects of 2-hydroxy-3-methylcyclopent-2-enone isolated from RH-MRP were demonstrated on AR, tyrosinase, and its antioxidant activity for the first time. RH-MRP and its constituents can be developed as beneficial functional food sources and cosmetic materials and should be investigated further as potential functional food sources.


