vibo-QuercitolCAS# 488-76-6 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 488-76-6 | SDF | Download SDF |
| PubChem ID | 441438 | Appearance | Powder |
| Formula | C6H12O5 | M.Wt | 164.2 |
| Type of Compound | Miscellaneous | Storage | Desiccate at -20°C |
| Solubility | Soluble in Chloroform,Dichloromethane,Ethyl Acetate,DMSO,Acetone,etc. | ||
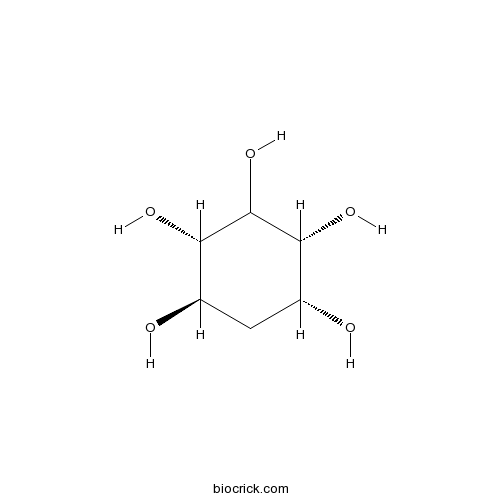
| Chemical Name | (1R,2S,4R,5R)-cyclohexane-1,2,3,4,5-pentol | ||
| SMILES | C1C(C(C(C(C1O)O)O)O)O | ||
| Standard InChIKey | IMPKVMRTXBRHRB-RSVSWTKNSA-N | ||
| Standard InChI | InChI=1S/C6H12O5/c7-2-1-3(8)5(10)6(11)4(2)9/h2-11H,1H2/t2-,3-,4-,5+,6?/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | (-)-vibo-Quercitol is a carbaglycosylamine glycosidase inhibitor. |
| Structure Identification | J Nat Prod. 2007 Mar;70(3):493-7. Epub 2007 Mar 9.Synthesis of valiolamine and some precursors for bioactive carbaglycosylamines from (-)-vibo-quercitol produced by biogenesis of myo-inositol.[Pubmed: 17346079]A convenient and practical synthesis of valiolamine (4) and its related carbaglycosylamine glycosidase inhibitors from (-)-vibo-Quercitol (13), a compound readily produced by biogenesis of myo-inositol (9), is described. Bioorg Med Chem Lett. 2011 Dec 1;21(23):7189-92.Transformation of quercitols into 4-methylenecyclohex-5-ene-1,2,3-triol derivatives, precursors for the chemical chaperones N-octyl-4-epi-β-valienamine (NOEV) and N-octyl-β-valienamine (NOV).[Pubmed: 22001090](+)-proto-Quercitol (1) and (-)-vibo-Quercitol (2), both of which could be readily prepared by the bioconversion of myo-inositol, were successfully converted into the corresponding 4-methylenecyclohex-5-ene-1,2,3-triol derivatives. These compounds were demonstrated to be suitable precursors, preserving their configurations, for bioactive carba-aminosugars such as the potent chemical chaperone drug candidates, N-octyl-4-epi-β-valienamine (NOEV, 3) and N-octyl-β-valienamine (NOV, 4). |

vibo-Quercitol Dilution Calculator

vibo-Quercitol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.0901 mL | 30.4507 mL | 60.9013 mL | 121.8027 mL | 152.2533 mL |
| 5 mM | 1.218 mL | 6.0901 mL | 12.1803 mL | 24.3605 mL | 30.4507 mL |
| 10 mM | 0.609 mL | 3.0451 mL | 6.0901 mL | 12.1803 mL | 15.2253 mL |
| 50 mM | 0.1218 mL | 0.609 mL | 1.218 mL | 2.4361 mL | 3.0451 mL |
| 100 mM | 0.0609 mL | 0.3045 mL | 0.609 mL | 1.218 mL | 1.5225 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Allitol
Catalog No.:BCN5593
CAS No.:488-44-8
- 3-Methylcatechol
Catalog No.:BCN3925
CAS No.:488-17-5
- Thesinine
Catalog No.:BCN1990
CAS No.:488-02-8
- Heleurine
Catalog No.:BCN1953
CAS No.:488-00-6
- Curcumol
Catalog No.:BCN5976
CAS No.:4871-97-0
- SNAP 94847 hydrochloride
Catalog No.:BCC7658
CAS No.:487051-12-7
- AR-A014418
Catalog No.:BCC1366
CAS No.:487021-52-3
- HI TOPK 032
Catalog No.:BCC6225
CAS No.:487020-03-1
- Lindelofine
Catalog No.:BCN2043
CAS No.:487-99-0
- Kainic acid
Catalog No.:BCC6572
CAS No.:487-79-6
- Hypaphorine
Catalog No.:BCN2775
CAS No.:487-58-1
- Butein
Catalog No.:BCN5592
CAS No.:487-52-5
- D-Ribitol-5-phosphate
Catalog No.:BCC4838
CAS No.:35320-17-3
- D-arabinitol
Catalog No.:BCN5595
CAS No.:488-82-4
- Furan-3-carboxylic acid
Catalog No.:BCN6397
CAS No.:488-93-7
- N-Acetylstepharine
Catalog No.:BCN7566
CAS No.:4880-87-9
- Elesclomol (STA-4783)
Catalog No.:BCC2337
CAS No.:488832-69-5
- Icariin
Catalog No.:BCN6311
CAS No.:489-32-7
- Limocitrin
Catalog No.:BCN3346
CAS No.:489-33-8
- Gossypetin
Catalog No.:BCN8075
CAS No.:489-35-0
- Globulol
Catalog No.:BCN6901
CAS No.:489-41-8
- Guaiazulen
Catalog No.:BCC8180
CAS No.:489-84-9
- Guaiol
Catalog No.:BCN6619
CAS No.:489-86-1
- ML 213
Catalog No.:BCC6213
CAS No.:489402-47-3
Transformation of quercitols into 4-methylenecyclohex-5-ene-1,2,3-triol derivatives, precursors for the chemical chaperones N-octyl-4-epi-beta-valienamine (NOEV) and N-octyl-beta-valienamine (NOV).[Pubmed:22001090]
Bioorg Med Chem Lett. 2011 Dec 1;21(23):7189-92.
(+)-proto-Quercitol (1) and (-)-vibo-Quercitol (2), both of which could be readily prepared by the bioconversion of myo-inositol, were successfully converted into the corresponding 4-methylenecyclohex-5-ene-1,2,3-triol derivatives. These compounds were demonstrated to be suitable precursors, preserving their configurations, for bioactive carba-aminosugars such as the potent chemical chaperone drug candidates, N-octyl-4-epi-beta-valienamine (NOEV, 3) and N-octyl-beta-valienamine (NOV, 4).
Synthesis of valiolamine and some precursors for bioactive carbaglycosylamines from (-)-vibo-quercitol produced by biogenesis of myo-inositol.[Pubmed:17346079]
J Nat Prod. 2007 Mar;70(3):493-7.
A convenient and practical synthesis of valiolamine (4) and its related carbaglycosylamine glycosidase inhibitors from (-)-vibo-Quercitol (13), a compound readily produced by biogenesis of myo-inositol (9), is described.


