D-Ribitol-5-phosphateCAS# 35320-17-3 |
- Amyloid β-Protein (1-15)
Catalog No.:BCC1003
CAS No.:183745-81-5
- Beta-Amyloid (1-11)
Catalog No.:BCC1002
CAS No.:190436-05-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 35320-17-3 | SDF | Download SDF |
| PubChem ID | 151104 | Appearance | Powder |
| Formula | C5H13O8P | M.Wt | 232.12 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | D-Ribitol 5-phosphate;Ribitol-5-phosphate;Ribitol 5-phosphate | ||
| Solubility | Soluble in DMSO > 10 mM | ||
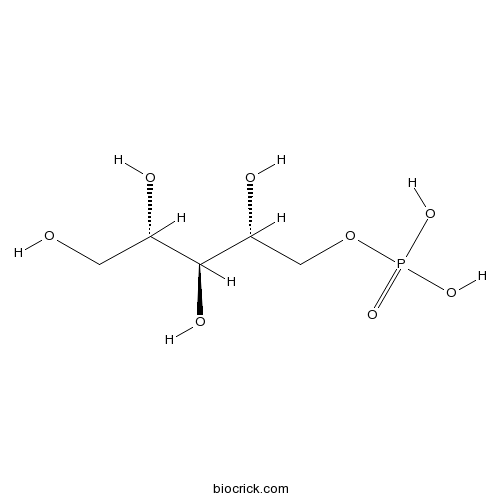
| Chemical Name | [(2R,3S,4S)-2,3,4,5-tetrahydroxypentyl] dihydrogen phosphate | ||
| SMILES | C(C(C(C(COP(=O)(O)O)O)O)O)O | ||
| Standard InChIKey | VJDOAZKNBQCAGE-LMVFSUKVSA-N | ||
| Standard InChI | InChI=1S/C5H13O8P/c6-1-3(7)5(9)4(8)2-13-14(10,11)12/h3-9H,1-2H2,(H2,10,11,12)/t3-,4+,5-/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Ribitol is a crystalline pentose alcohol formed by the reduction of ribose. Enhancing the flux of D-glucose to the pentose phosphate pathway in Saccharomyces cerevisiae for the production of D-ribose and ribitol.In Vitro:Ribitol is a reduced sugar[1]. Phosphoglucose isomerase-deficient (pgi1) strains of Saccharomyces cerevisiae are studied for the production of D-ribose and Ribitol from D-glucose via the intermediates of the pentose phosphate pathway. Overexpression of the gene encoding sugar phosphate phosphatase (DOG1) of S. cerevisiae is needed for the production of D-ribose and Ribitol. The engineered strains are compared for their ability to produce the PPP-derived 5-carbon compounds Ribitol and D-ribose from D-glucose[2]. References: | |||||

D-Ribitol-5-phosphate Dilution Calculator

D-Ribitol-5-phosphate Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.3081 mL | 21.5406 mL | 43.0812 mL | 86.1623 mL | 107.7029 mL |
| 5 mM | 0.8616 mL | 4.3081 mL | 8.6162 mL | 17.2325 mL | 21.5406 mL |
| 10 mM | 0.4308 mL | 2.1541 mL | 4.3081 mL | 8.6162 mL | 10.7703 mL |
| 50 mM | 0.0862 mL | 0.4308 mL | 0.8616 mL | 1.7232 mL | 2.1541 mL |
| 100 mM | 0.0431 mL | 0.2154 mL | 0.4308 mL | 0.8616 mL | 1.077 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Ribitol is a crystalline pentose alcohol and is formed by the reduction of ribose which is occurs naturally in the plant Adonis vernalis.
- vibo-Quercitol
Catalog No.:BCN5594
CAS No.:488-76-6
- Allitol
Catalog No.:BCN5593
CAS No.:488-44-8
- 3-Methylcatechol
Catalog No.:BCN3925
CAS No.:488-17-5
- Thesinine
Catalog No.:BCN1990
CAS No.:488-02-8
- Heleurine
Catalog No.:BCN1953
CAS No.:488-00-6
- Curcumol
Catalog No.:BCN5976
CAS No.:4871-97-0
- SNAP 94847 hydrochloride
Catalog No.:BCC7658
CAS No.:487051-12-7
- AR-A014418
Catalog No.:BCC1366
CAS No.:487021-52-3
- HI TOPK 032
Catalog No.:BCC6225
CAS No.:487020-03-1
- Lindelofine
Catalog No.:BCN2043
CAS No.:487-99-0
- Kainic acid
Catalog No.:BCC6572
CAS No.:487-79-6
- Hypaphorine
Catalog No.:BCN2775
CAS No.:487-58-1
- D-arabinitol
Catalog No.:BCN5595
CAS No.:488-82-4
- Furan-3-carboxylic acid
Catalog No.:BCN6397
CAS No.:488-93-7
- N-Acetylstepharine
Catalog No.:BCN7566
CAS No.:4880-87-9
- Elesclomol (STA-4783)
Catalog No.:BCC2337
CAS No.:488832-69-5
- Icariin
Catalog No.:BCN6311
CAS No.:489-32-7
- Limocitrin
Catalog No.:BCN3346
CAS No.:489-33-8
- Gossypetin
Catalog No.:BCN8075
CAS No.:489-35-0
- Globulol
Catalog No.:BCN6901
CAS No.:489-41-8
- Guaiazulen
Catalog No.:BCC8180
CAS No.:489-84-9
- Guaiol
Catalog No.:BCN6619
CAS No.:489-86-1
- ML 213
Catalog No.:BCC6213
CAS No.:489402-47-3
- SBC-115076
Catalog No.:BCC6440
CAS No.:489415-96-5
ISPD produces CDP-ribitol used by FKTN and FKRP to transfer ribitol phosphate onto alpha-dystroglycan.[Pubmed:27194101]
Nat Commun. 2016 May 19;7:11534.
Mutations in genes required for the glycosylation of alpha-dystroglycan lead to muscle and brain diseases known as dystroglycanopathies. However, the precise structure and biogenesis of the assembled glycan are not completely understood. Here we report that three enzymes mutated in dystroglycanopathies can collaborate to attach ribitol phosphate onto alpha-dystroglycan. Specifically, we demonstrate that isoprenoid synthase domain-containing protein (ISPD) synthesizes CDP-ribitol, present in muscle, and that both recombinant fukutin (FKTN) and fukutin-related protein (FKRP) can transfer a ribitol phosphate group from CDP-ribitol to alpha-dystroglycan. We also show that ISPD and FKTN are essential for the incorporation of ribitol into alpha-dystroglycan in HEK293 cells. Glycosylation of alpha-dystroglycan in fibroblasts from patients with hypomorphic ISPD mutations is reduced. We observe that in some cases glycosylation can be partially restored by addition of ribitol to the culture medium, suggesting that dietary supplementation with ribitol should be evaluated as a therapy for patients with ISPD mutations.
The Muscular Dystrophy Gene TMEM5 Encodes a Ribitol beta1,4-Xylosyltransferase Required for the Functional Glycosylation of Dystroglycan.[Pubmed:27733679]
J Biol Chem. 2016 Nov 18;291(47):24618-24627.
A defect in O-mannosyl glycan is the cause of alpha-dystroglycanopathy, a group of congenital muscular dystrophies caused by aberrant alpha-dystroglycan (alpha-DG) glycosylation. Recently, the entire structure of O-mannosyl glycan, [3GlcAbeta1-3Xylalpha1]n-3GlcAbeta1-4Xyl-Rbo5P-1Rbo5P-3GalNAcbeta1-3GlcNAcbeta1-4 (phospho-6)Manalpha1-, which is required for the binding of alpha-DG to extracellular matrix ligands, has been proposed. However, the linkage of the first Xyl residue to ribitol 5-phosphate (Rbo5P) is not clear. TMEM5 is a gene product responsible for alpha-dystroglycanopathy and was reported as a potential enzyme involved in this linkage formation, although the experimental evidence is still incomplete. Here, we report that TMEM5 is a xylosyltransferase that forms the Xylbeta1-4Rbo5P linkage on O-mannosyl glycan. The anomeric configuration and linkage position of the product (beta1,4 linkage) was determined by NMR analysis. The introduction of two missense mutations in TMEM5 found in alpha-dystroglycanopathy patients impaired xylosyltransferase activity. Furthermore, the disruption of the TMEM5 gene by CRISPR/Cas9 abrogated the elongation of the (-3GlcAbeta1-3Xylalpha1-) unit on O-mannosyl glycan. Based on these results, we concluded that TMEM5 acts as a UDP-d-xylose:ribitol-5-phosphate beta1,4-xylosyltransferase in the biosynthetic pathway of O-mannosyl glycan.
The functional O-mannose glycan on alpha-dystroglycan contains a phospho-ribitol primed for matriglycan addition.[Pubmed:27130732]
Elife. 2016 Apr 29;5.
Multiple glycosyltransferases are essential for the proper modification of alpha-dystroglycan, as mutations in the encoding genes cause congenital/limb-girdle muscular dystrophies. Here we elucidate further the structure of an O-mannose-initiated glycan on alpha-dystroglycan that is required to generate its extracellular matrix-binding polysaccharide. This functional glycan contains a novel ribitol structure that links a phosphotrisaccharide to xylose. ISPD is a CDP-ribitol (ribose) pyrophosphorylase that generates the reduced sugar nucleotide for the insertion of ribitol in a phosphodiester linkage to the glycoprotein. TMEM5 is a UDP-xylosyl transferase that elaborates the structure. We demonstrate in a zebrafish model as well as in a human patient that defects in TMEM5 result in muscular dystrophy in combination with abnormal brain development. Thus, we propose a novel structure-a ribitol in a phosphodiester linkage-for the moiety on which TMEM5, B4GAT1, and LARGE act to generate the functional receptor for ECM proteins having LG domains.
Immunoassays for riboflavin and flavin mononucleotide using antibodies specific to d-ribitol and d-ribitol-5-phosphate.[Pubmed:28327345]
J Immunol Methods. 2017 Jun;445:59-66.
Riboflavin (vitamin B2), a water-soluble vitamin, plays a key role in maintaining human health. Though, numerous methods have been reported for the determination of total riboflavin (TRF) content in foods and biological samples, very few methods are reported for quantifying riboflavin and its coenzymes [flavin mononucleotide (FMN); flavin adenine dinucleotide (FAD)] individually. Recently, we have demonstrated that antibodies specific to d-ribitol and D-Ribitol-5-phosphate also recognize riboflavin and FMN, respectively, and not vice-versa. In this study, we have evaluated these two antibodies for the analysis of riboflavin and FMN by indirect competitive ELISA (icELISA) in selected foods and pharmaceuticals. Under the optimal assay conditions, 50% inhibition concentration (IC50) and limit of detection (LOD, IC10) were 3.41ng/mL and 0.02ng/mL for riboflavin, and 7.84ng/mL and 0.24ng/mL for FMN, respectively, with detectable concentration range between 0.1 and 100ng of analytes and <0.1% cross-reactivity with other water-soluble vitamins. The amounts of TRF in food samples, as analyzed by icELISA using ribitol antibody, were 90-95% of the reported values in the literature or label values. Quantification of individual flavins (riboflavin and FMN) from the same food samples showed variation in their values compared to TRF, and were in good agreement with values obtained from HPLC and AOAC methods. Further, spiking and recovery analysis of food samples and pharmaceuticals showed no significant matrix effects. The immunoassays were validated in terms of accuracy and precision using inter- and intra-assays. The immunoassays developed in this study are sensitive and appears feasible for screening a large number of samples in the quantification of riboflavin and FMN in various biological samples, pharmaceuticals and natural/processed foods.


