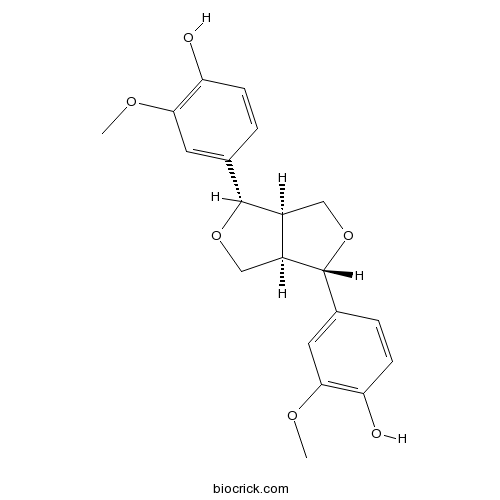
PinoresinolCAS# 487-36-5 |
- (-)-Epipinoresinol
Catalog No.:BCN3377
CAS No.:10061-38-8
- (+)-Epipinoresinol
Catalog No.:BCN3255
CAS No.:24404-50-0
- (-)-Pinoresinol
Catalog No.:BCN3254
CAS No.:81446-29-9
- (±)-Pinoresinol
Catalog No.:BCN9179
CAS No.:4263-88-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 487-36-5 | SDF | Download SDF |
| PubChem ID | 73399 | Appearance | White-beige powder |
| Formula | C20H22O6 | M.Wt | 358.4 |
| Type of Compound | Lignans | Storage | Desiccate at -20°C |
| Solubility | Soluble in methan | ||
| Chemical Name | 4-[(3S,3aR,6S,6aR)-6-(4-hydroxy-3-methoxyphenyl)-1,3,3a,4,6,6a-hexahydrofuro[3,4-c]furan-3-yl]-2-methoxyphenol | ||
| SMILES | COC1=C(C=CC(=C1)C2C3COC(C3CO2)C4=CC(=C(C=C4)O)OC)O | ||
| Standard InChIKey | HGXBRUKMWQGOIE-AFHBHXEDSA-N | ||
| Standard InChI | InChI=1S/C20H22O6/c1-23-17-7-11(3-5-15(17)21)19-13-9-26-20(14(13)10-25-19)12-4-6-16(22)18(8-12)24-2/h3-8,13-14,19-22H,9-10H2,1-2H3/t13-,14-,19+,20+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Pinoresinol has antiinflammatory, hepatoprotective, and fungicidal activities, it can protect pial microcirculation from I-reperfusion injury, to increase nitric oxide release and to reduce oxidative stress preserving pial blood flow distribution; it may exert pharmacologically interesting effects via modulation of the insulin-like signalling pathway in C. elegans. Pinoresinol causes an upregulation of the CDK inhibitor p21(WAF1/Cip1) both at mRNA and protein levels, inhibits NF-kappaB and activating protein 1 (AP-1). |
| Targets | p53 | CDK | p21 | NF-kB | COX | PGE | IL Receptor | AP-1 | Antifection |
| In vitro | Among plant lignans, pinoresinol has the strongest antiinflammatory properties in human intestinal Caco-2 cells.[Pubmed: 22955517]J Nutr. 2012 Oct;142(10):1798-805.Dietary lignans show some promising health benefits, but little is known about their fate and activities in the small intestine. The purpose of this study was thus to investigate whether plant lignans are taken up by intestinal cells and modulate the intestinal inflammatory response using the Caco-2 cell model.
Antifungal effect of (+)-pinoresinol isolated from Sambucus williamsii.[Pubmed: 20657496 ]Molecules. 2010 May 14;15(5):3507-16.In this study, we investigated the antifungal activity and mechanism of action of (+)-Pinoresinol, a biphenolic compound isolated from the herb Sambucus williamsii,used in traditional medicine.
(+)-Pinoresinol displays potent antifungal properties without hemolytic effects on human erythrocytes. |
| In vivo | The Lignan Pinoresinol Induces Nuclear Translocation of DAF-16 in Caenorhabditis elegans but has No Effect on Life Span.[Pubmed: 25826281]Phytother Res. 2015 Jun;29(6):894-901.The lignan Pinoresinol is a constituent of flaxseed, sesame seeds and olive oil. Because of different molecular effects reported for this compound, e.g. antioxidative activity, Pinoresinol is suggested to cause positive effects on humans.
|
| Cell Research | Pinoresinol inhibits proliferation and induces differentiation on human HL60 leukemia cells.[Pubmed: 24099079]Nutr Cancer. 2013;65(8):1208-18.Pinoresinol (PIN), one of the simplest lignans, is the precursor of other dietary lignans that are present in whole-grain cereals, legumes, fruits, and other vegetables.
Several experimental and epidemiological evidences suggest that lignans may prevent human cancer in different organs.
|
| Animal Research | Hepatoprotective effect of pinoresinol on carbon tetrachloride-induced hepatic damage in mice.[Pubmed: 20093790]Effects of oleuropein and pinoresinol on microvascular damage induced by hypoperfusion and reperfusion in rat pial circulation.[Pubmed: 25243351]Microcirculation. 2015 Jan;22(1):79-90.The present study was aimed to assess the in vivo acute effects of oleuropein or/and Pinoresinol, polyphenols widely diffused in natural sources, on rat pial microvascular responses during transient BCCAO and reperfusion.
J Pharmacol Sci. 2010;112(1):105-12.Forsythiae Fructus is known to have diuretic, anti-bacterial, and anti-inflammatory activities. This study examined the hepatoprotective effects of Pinoresinol, a lignan isolated from Forsythiae Fructus, against carbon tetrachloride (CCl(4))-induced liver injury.
|

Pinoresinol Dilution Calculator

Pinoresinol Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.7902 mL | 13.9509 mL | 27.9018 mL | 55.8036 mL | 69.7545 mL |
| 5 mM | 0.558 mL | 2.7902 mL | 5.5804 mL | 11.1607 mL | 13.9509 mL |
| 10 mM | 0.279 mL | 1.3951 mL | 2.7902 mL | 5.5804 mL | 6.9754 mL |
| 50 mM | 0.0558 mL | 0.279 mL | 0.558 mL | 1.1161 mL | 1.3951 mL |
| 100 mM | 0.0279 mL | 0.1395 mL | 0.279 mL | 0.558 mL | 0.6975 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Syringaresinol
Catalog No.:BCN3042
CAS No.:487-35-4
- Scopoline
Catalog No.:BCN1942
CAS No.:487-27-4
- Elemicin
Catalog No.:BCN2818
CAS No.:487-11-6
- Citropten
Catalog No.:BCN4831
CAS No.:487-06-9
- Cyclocalopin A
Catalog No.:BCN5587
CAS No.:486430-94-8
- O-Acetylcyclocalopin A
Catalog No.:BCN5586
CAS No.:486430-93-7
- CIQ
Catalog No.:BCC7862
CAS No.:486427-17-2
- AZD2858
Catalog No.:BCC4509
CAS No.:486424-20-8
- Thermopsine
Catalog No.:BCN2603
CAS No.:486-90-8
- Anagyrine
Catalog No.:BCN3049
CAS No.:486-89-5
- alpha-Isolupanine
Catalog No.:BCN7989
CAS No.:486-87-3
- N-Methylcytisine
Catalog No.:BCN1266
CAS No.:486-86-2
- Phillygenin
Catalog No.:BCN2653
CAS No.:487-39-8
- Phillyrin
Catalog No.:BCN1096
CAS No.:487-41-2
- Ononetin
Catalog No.:BCC6367
CAS No.:487-49-0
- Butein
Catalog No.:BCN5592
CAS No.:487-52-5
- Hypaphorine
Catalog No.:BCN2775
CAS No.:487-58-1
- Kainic acid
Catalog No.:BCC6572
CAS No.:487-79-6
- Lindelofine
Catalog No.:BCN2043
CAS No.:487-99-0
- HI TOPK 032
Catalog No.:BCC6225
CAS No.:487020-03-1
- AR-A014418
Catalog No.:BCC1366
CAS No.:487021-52-3
- SNAP 94847 hydrochloride
Catalog No.:BCC7658
CAS No.:487051-12-7
- Curcumol
Catalog No.:BCN5976
CAS No.:4871-97-0
- Heleurine
Catalog No.:BCN1953
CAS No.:488-00-6
Effects of oleuropein and pinoresinol on microvascular damage induced by hypoperfusion and reperfusion in rat pial circulation.[Pubmed:25243351]
Microcirculation. 2015 Jan;22(1):79-90.
OBJECTIVE: The present study was aimed to assess the in vivo acute effects of oleuropein or/and Pinoresinol, polyphenols widely diffused in natural sources, on rat pial microvascular responses during transient BCCAO and reperfusion. METHODS: Rat pial microcirculation was visualized by fluorescence microscopy through a closed cranial window. Pial arterioles were classified into five orders of branching. Capillaries were assigned order 0, the smallest arterioles order 1 and the largest ones order 5. RESULTS: Rats subjected to BCCAO and reperfusion showed: arteriolar diameter decrease, microvascular leakage, leukocyte adhesion in venules, and reduction in capillary perfusion. Pretreatment with oleuropein or Pinoresinol, a higher dose before BCCAO determined dilation in all arteriolar orders RE. Microvascular leakage was reduced as well as leukocyte adhesion and ROS formation, while capillary perfusion was protected. Inhibition of endothelium nitric oxide synthase prior to oleuropein or Pinoresinol reduced the effect of these polyphenols on pial arteriolar diameter and leakage. These substances, administered together, prevented microvascular damage to a larger extent. CONCLUSION: Oleuropein and Pinoresinol were both able to protect pial microcirculation from I-reperfusion injury, to increase nitric oxide release and to reduce oxidative stress preserving pial blood flow distribution.
Among plant lignans, pinoresinol has the strongest antiinflammatory properties in human intestinal Caco-2 cells.[Pubmed:22955517]
J Nutr. 2012 Oct;142(10):1798-805.
Dietary lignans show some promising health benefits, but little is known about their fate and activities in the small intestine. The purpose of this study was thus to investigate whether plant lignans are taken up by intestinal cells and modulate the intestinal inflammatory response using the Caco-2 cell model. Six lignan standards [secoisolariciresinol diglucoside (SDG), secoisolariciresinol (SECO), Pinoresinol (PINO), lariciresinol, matairesinol (MAT), and hydroxymatairesinol] and their colonic metabolites [enterolactone (ENL) and enterodiol] were studied. First, differentiated cells were exposed to SDG, SECO, PINO, or ENL at increasing concentrations for 4 h, and their cellular contents (before and after deconjugation) were determined by HPLC. Second, in IL-1beta-stimulated confluent and/or differentiated cells, lignan effects were tested on different soluble proinflammatory mediators quantified by enzyme immunoassays and on the NF-kappaB activation pathway by using cells transiently transfected. SECO, PINO, and ENL, but not SDG, were taken up and partly conjugated by cells, which is a saturable conjugation process. PINO was the most efficiently conjugated (75% of total in cells). In inflamed cells, PINO significantly reduced IL-6 by 65% and 30% in confluent and differentiated cells, respectively, and cyclooxygenase (COX)-2-derived prostaglandin E(2) by 62% in confluent cells. In contrast, MAT increased significantly COX-2-derived prostaglandin E(2) in confluent cells. Moreover, PINO dose-dependently decreased IL-6 and macrophage chemoattractant protein-1 secretions and NF-kappaB activity. Our findings suggest that plant lignans can be absorbed and metabolized in the small intestine and, among the plant lignans tested, PINO exhibited the strongest antiinflammatory properties by acting on the NF-kappaB signaling pathway, possibly in relation to its furofuran structure and/or its intestinal metabolism.
The Lignan Pinoresinol Induces Nuclear Translocation of DAF-16 in Caenorhabditis elegans but has No Effect on Life Span.[Pubmed:25826281]
Phytother Res. 2015 Jun;29(6):894-901.
The lignan Pinoresinol is a constituent of flaxseed, sesame seeds and olive oil. Because of different molecular effects reported for this compound, e.g. antioxidative activity, Pinoresinol is suggested to cause positive effects on humans. Because experimental data are limited, we have analysed the effects of the lignan on the nematode Caenorhabditis elegans: in spite of a strong antioxidative capacity detected in an in vitro assay, no antioxidative effects were detectable in vivo. In analogy to this result, no modulation of the sensitivity against thermal stress was detectable. However, incubation with Pinoresinol caused an enhanced nuclear accumulation of the transcription factor DAF-16 (insulin/IGF-like signalling pathway). Using a strain with an enhanced oxidative stress level (mev-1 mutant), we clearly see an increase in stress resistance caused by this lignan, but no change in reactive oxygen species. Furthermore, we investigated the effects of Pinoresinol on the life span of the nematode, but no modulation was found, neither in wild-type nor in mev-1 mutant nematodes. These results suggest that Pinoresinol may exert pharmacologically interesting effects via modulation of the insulin-like signalling pathway in C. elegans as well as in other species like mammals due to the evolutionary conservation of this signalling pathway.
Hepatoprotective effect of pinoresinol on carbon tetrachloride-induced hepatic damage in mice.[Pubmed:20093790]
J Pharmacol Sci. 2010;112(1):105-12.
Forsythiae Fructus is known to have diuretic, anti-bacterial, and anti-inflammatory activities. This study examined the hepatoprotective effects of Pinoresinol, a lignan isolated from Forsythiae Fructus, against carbon tetrachloride (CCl(4))-induced liver injury. Mice were treated intraperitoneally with vehicle or Pinoresinol (25, 50, 100, and 200 mg/kg) 30 min before and 2 h after CCl4 (20 microl/kg) injection. In the vehicle-treated CCl(4 )group, serum aminotransferase activities were significantly increased 24 h after CCl4 injection, and these increases were attenuated by Pinoresinol at all doses. Hepatic glutathione contents were significantly decreased and lipid peroxidation was increased after CCl4 treatment. These changes were attenuated by 50 and 100 mg/kg of Pinoresinol. The levels of protein and mRNA expression of inflammatory mediators, including tumor necrosis factor-alpha, inducible nitric oxide synthase, and cyclooxygenase-2, were significantly increased after CCl4 injection; and these increases were attenuated by Pinoresinol. Nuclear translocation of nuclear factor-kappaB (NF-kappaB) and phosphorylation of c-Jun, one of the components of activating protein 1 (AP-1), were inhibited by Pinoresinol. Our results suggest that Pinoresinol ameliorates CCl4)-induced acute liver injury, and this protection is likely due to anti-oxidative activity and down-regulation of inflammatory mediators through inhibition of NF-kappaB and AP-1.
Pinoresinol inhibits proliferation and induces differentiation on human HL60 leukemia cells.[Pubmed:24099079]
Nutr Cancer. 2013;65(8):1208-18.
Pinoresinol (PIN), one of the simplest lignans, is the precursor of other dietary lignans that are present in whole-grain cereals, legumes, fruits, and other vegetables. Several experimental and epidemiological evidences suggest that lignans may prevent human cancer in different organs. In this study we investigated the chemopreventive properties of PIN on cell lines derived from different sites either expressing or not the functional tumor suppressor protein p53. It was found that PIN inhibited the proliferation of p53 wild type colon and prostate tumor cells (HCT116 and LNCaP) while in breast cells the inhibition of growth was observed only in p53 mutant cells (MDA-MB-231). A potent antiproliferative activity of PIN was also observed on p53 null cells HL60 (IC50% 8 muM), their multidrug resistant variant HL60R (IC50% 32 muM) and K562. On HL60 cells, PIN caused a block of cell cycle in the G0/G1 phase, induced a weak proapoptotic effect but it was a good trigger of differentiation (NBT reduction and CD11b expression). PIN caused an upregulation of the CDK inhibitor p21(WAF1/Cip1) both at mRNA and protein levels so suggesting that this could be a mechanism by which PIN reduced proliferation and induced differentiation on HL60 cells.
Antifungal effect of (+)-pinoresinol isolated from Sambucus williamsii.[Pubmed:20657496]
Molecules. 2010 May 14;15(5):3507-16.
In this study, we investigated the antifungal activity and mechanism of action of (+)-Pinoresinol, a biphenolic compound isolated from the herb Sambucus williamsii,used in traditional medicine. (+)-Pinoresinol displays potent antifungal properties without hemolytic effects on human erythrocytes. To understand the antifungal mechanism of (+)-Pinoresinol, we conducted fluorescence experiments on the human pathogen Candida albicans. Fluorescence analysis using 1,6-diphenyl-1,3,5-hexatriene (DPH) indicated that the (+)-Pinoresinol caused damage to the fungal plasma membrane. This result was confirmed by using rhodamine-labeled giant unilamellar vesicle (GUV) experiments. Therefore, the present study indicates that (+)-Pinoresinol possesses fungicidal activities and therapeutic potential as an antifungal agent for the treatment of fungal infectious diseases in humans.


