MY-5445PDE5 inhibitor CAS# 78351-75-4 |
- PSI-7977
Catalog No.:BCC1871
CAS No.:1190307-88-0
- Trovafloxacin mesylate
Catalog No.:BCC3931
CAS No.:147059-75-4
- Capecitabine
Catalog No.:BCN2168
CAS No.:154361-50-9
- Viomycin
Catalog No.:BCC3930
CAS No.:32988-50-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 78351-75-4 | SDF | Download SDF |
| PubChem ID | 1348 | Appearance | Powder |
| Formula | C20H14ClN3 | M.Wt | 331.8 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 50 mM in DMSO and to 5 mM in ethanol | ||
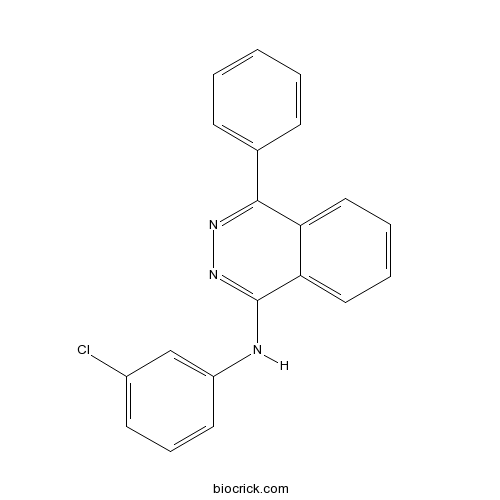
| Chemical Name | N-(3-chlorophenyl)-4-phenylphthalazin-1-amine | ||
| SMILES | C1=CC=C(C=C1)C2=NN=C(C3=CC=CC=C32)NC4=CC(=CC=C4)Cl | ||
| Standard InChIKey | CEHQLKSLMFIHBF-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C20H14ClN3/c21-15-9-6-10-16(13-15)22-20-18-12-5-4-11-17(18)19(23-24-20)14-7-2-1-3-8-14/h1-13H,(H,22,24) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Specific inhibitor of cyclic GMP phosphodiesterase, selective for PDE5 (IC50 = 0.5 μM). |

MY-5445 Dilution Calculator

MY-5445 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0139 mL | 15.0693 mL | 30.1386 mL | 60.2773 mL | 75.3466 mL |
| 5 mM | 0.6028 mL | 3.0139 mL | 6.0277 mL | 12.0555 mL | 15.0693 mL |
| 10 mM | 0.3014 mL | 1.5069 mL | 3.0139 mL | 6.0277 mL | 7.5347 mL |
| 50 mM | 0.0603 mL | 0.3014 mL | 0.6028 mL | 1.2055 mL | 1.5069 mL |
| 100 mM | 0.0301 mL | 0.1507 mL | 0.3014 mL | 0.6028 mL | 0.7535 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Pyranojacareubin
Catalog No.:BCN7429
CAS No.:78343-62-1
- Nocamycin I
Catalog No.:BCN1845
CAS No.:78339-49-8
- PCI-24781 (CRA-024781)
Catalog No.:BCC2155
CAS No.:783355-60-2
- MLN120B
Catalog No.:BCC1772
CAS No.:783348-36-7
- MRK 016
Catalog No.:BCC6070
CAS No.:783331-24-8
- H-D-1-Nal-OH
Catalog No.:BCC3281
CAS No.:78306-92-0
- 7-Ethylcamptothecin
Catalog No.:BCN2480
CAS No.:78287-27-1
- Ecliptasaponin A
Catalog No.:BCN3843
CAS No.:78285-90-2
- Nepafenac
Catalog No.:BCC1258
CAS No.:78281-72-8
- Hydroxysafflor yellow A
Catalog No.:BCN1049
CAS No.:78281-02-4
- [Orn5]-URP
Catalog No.:BCC5985
CAS No.:782485-03-4
- Paroxetine HCl
Catalog No.:BCC5054
CAS No.:78246-49-8
- 1,7-Dihydroxy-2,3-dimethoxyxanthone
Catalog No.:BCN7523
CAS No.:78405-33-1
- Milrinone
Catalog No.:BCC4374
CAS No.:78415-72-2
- Trequinsin hydrochloride
Catalog No.:BCC7333
CAS No.:78416-81-6
- 5,7,3'-Trihydroxy-6,4',5'-trimethoxyflavone
Catalog No.:BCN1353
CAS No.:78417-26-2
- RGB-286638
Catalog No.:BCC5519
CAS No.:784210-87-3
- RGB-286638 free base
Catalog No.:BCC5520
CAS No.:784210-88-4
- Deacetyltaxol
Catalog No.:BCN2820
CAS No.:78432-77-6
- 19-Hydroxybaccatin III
Catalog No.:BCN4330
CAS No.:78432-78-7
- 7-Epi 10-Desacetyl Paclitaxel
Catalog No.:BCC1314
CAS No.:78454-17-8
- Enterolakton
Catalog No.:BCC8170
CAS No.:78473-71-9
- Praeruptorin E
Catalog No.:BCN2591
CAS No.:78478-28-1
- 7-Epi-10-deacetylcephalomannine
Catalog No.:BCN7673
CAS No.:78479-12-6
Effect of 1-(3-chloroanilino)-4-phenylphthalazine (MY-5445), a specific inhibitor of cyclic GMP phosphodiesterase, on human platelet aggregation.[Pubmed:6141286]
J Pharmacol Exp Ther. 1984 Feb;228(2):467-71.
The effects of a novel compound, 1-(3-chloroanilino)-4-phenylphthalazine (MY-5445), on cyclic nucleotide metabolism and in vitro aggregation of human platelets were investigated. The concentrations of MY-5445 producing 50% inhibition of human platelet aggregation induced by 3 microM ADP, 3 micrograms/ml of collagen and 100 micrograms/ml of arachidonic acid were 0.07, 0.02 and 0.17 microM, respectively. Addition of MY-5445 significantly elevated cyclic GMP content in human platelets but had no effect on cyclic AMP content, suggesting that the drug affects principally the cyclic GMP metabolism in the platelet. Although MY-5445 had no effect on either adenylate cyclase or guanylate cyclase activity, it inhibited specifically human platelet cyclic GMP phosphodiesterase which was separated from cyclic AMP phosphodiesterase by diethylaminoethyl-cellulose column chromatography. The inhibitory effect of MY-5445 on cyclic GMP phosphodiesterase was also demonstrated by direct binding of the enzyme to MY-5445 coupled Sepharose, which was a useful tool for purifying the cyclic GMP phosphodiesterase from human platelet. These results would suggest that MY-5445 inhibits human platelet aggregation by increasing cyclic GMP content and that it provides a useful probe for elucidating the role of cyclic GMP in platelet aggregation.
Role of selective cyclic GMP phosphodiesterase inhibition in the myorelaxant actions of M&B 22,948, MY-5445, vinpocetine and 1-methyl-3-isobutyl-8-(methylamino)xanthine.[Pubmed:2480168]
Br J Pharmacol. 1989 Nov;98(3):725-34.
1. The mechanism by which M&B 22,948, MY-5445, vinpocetine and 1-methyl-3-isobutyl-8-(methylamino)xanthine (MIMAX), which have been described as selective cyclic GMP phosphodiesterase (PDE) inhibitors, relax rat aorta was investigated. 2. Three cyclic nucleotide PDEs were identified in the soluble fraction of rat aorta; a Ca2+-insensitive form exhibiting substrate selectivity for cyclic GMP (cGMP PDE), a Ca2+/calmodulin-stimulated form which also preferentially hydrolyzed cyclic GMP (Ca2+ PDE), and a form demonstrating substrate selectivity for cyclic AMP (cAMP PDE). 3. M&B 22,948 and MIMAX inhibited cGMP PDE (Ki = 0.16 microM and 0.43 microM, respectively) and Ca2+ PDE (Ki = 9.9 microM and 0.55 microM, respectively), but exhibited weak activity against cAMP PDE (Ki = 249 microM and 42 microM, respectively). MY-5445 selectivity inhibited cGMP PDE (Ki = 1.3 microM) and vinpocetine selectively inhibited Ca2+ PDE (Ki = 14 microM). 4. M&B 22,948 and MIMAX induced dose-dependent increases in the accumulation of cyclic GMP, but not cyclic AMP, in rat aorta pieces. These effects were greatly reduced by endothelial denudation and by methylene blue (5 microM) which blocks the actions of endothelium-derived relaxant factor. MY-5445 and vinpocetine had no effect on rat aorta cyclic GMP or cyclic AMP accumulation. 5. All four compounds caused dose-related relaxation of 5-hydroxytryptamine (10 microM) contracted, endothelium-intact rat aorta, the effects of M&B 22,948 and MIMAX being greatly reduced by methylene blue (5 microM). Methylene blue also caused 10 fold and 100 fold rightward shifts in the dose-response curves of MY-5445 and vinpocetine, respectively. 6. The results are consistent with the smooth muscle relaxant actions of M&B 22,948 and MIMAX, but not vinpocetine and MY-5445, being mediated through a mechanism involving inhibition of cyclic GMP hydrolysis.


