Mepenzolate BromidemAChR antagonist CAS# 76-90-4 |
- NQDI 1
Catalog No.:BCC2404
CAS No.:175026-96-7
- GRI 977143
Catalog No.:BCC2401
CAS No.:325850-81-5
- Mdivi 1
Catalog No.:BCC2402
CAS No.:338967-87-6
- DAPK Substrate Peptide
Catalog No.:BCC2400
CAS No.:386769-53-5
- Cesium chloride
Catalog No.:BCC2399
CAS No.:7647-17-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 76-90-4 | SDF | Download SDF |
| PubChem ID | 6461 | Appearance | Powder |
| Formula | C21H26BrNO3 | M.Wt | 420.34 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | >20.7mg/mL in DMSO | ||
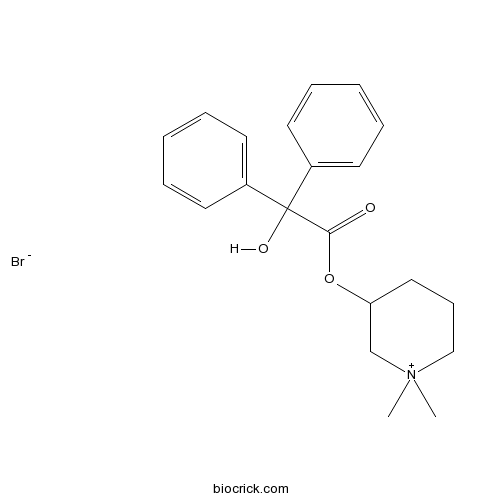
| Chemical Name | (1,1-dimethylpiperidin-1-ium-3-yl) 2-hydroxy-2,2-diphenylacetate;bromide | ||
| SMILES | C[N+]1(CCCC(C1)OC(=O)C(C2=CC=CC=C2)(C3=CC=CC=C3)O)C.[Br-] | ||
| Standard InChIKey | JRRNZNSGDSFFIR-UHFFFAOYSA-M | ||
| Standard InChI | InChI=1S/C21H26NO3.BrH/c1-22(2)15-9-14-19(16-22)25-20(23)21(24,17-10-5-3-6-11-17)18-12-7-4-8-13-18;/h3-8,10-13,19,24H,9,14-16H2,1-2H3;1H/q+1;/p-1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Mepenzolate Bromide is a muscarinic antagonist used to treat gastrointestinal disorders, decreases the severity of elastase-induced airspace enlargement and respiratory dysfunction.
IC50 value:
Target: mAChR antagonist
Oral administration of mepenzolate caused not only bronchodilation but also decreased the severity of elastase-induced pulmonary emphysema; however, compared with the intratracheal route of administration, about 5000 times higher dose was required to achieve this effect. Intravenously or intrarectally administered mepenzolate also showed these pharmacological effects. The intratracheal route of mepenzolate administration, but not other routes, resulted in protective effects against elastase-induced pulmonary damage and bronchodilation at a much lower dose than that which affected defecation and heart rate. References: | |||||

Mepenzolate Bromide Dilution Calculator

Mepenzolate Bromide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.379 mL | 11.8951 mL | 23.7903 mL | 47.5805 mL | 59.4757 mL |
| 5 mM | 0.4758 mL | 2.379 mL | 4.7581 mL | 9.5161 mL | 11.8951 mL |
| 10 mM | 0.2379 mL | 1.1895 mL | 2.379 mL | 4.7581 mL | 5.9476 mL |
| 50 mM | 0.0476 mL | 0.2379 mL | 0.4758 mL | 0.9516 mL | 1.1895 mL |
| 100 mM | 0.0238 mL | 0.119 mL | 0.2379 mL | 0.4758 mL | 0.5948 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Mepenzolate bromide occurs as a white or light cream-colored powder, which is freely soluble in methanol, slightly soluble in water and chloroform, and practically insoluble in ether.
- Trityl Chloride
Catalog No.:BCC2805
CAS No.:76-83-5
- Tephrosin
Catalog No.:BCN4742
CAS No.:76-80-2
- Quassin
Catalog No.:BCN4315
CAS No.:76-78-8
- Neoquassine
Catalog No.:BCN3120
CAS No.:76-77-7
- Rhynchophylline
Catalog No.:BCN4979
CAS No.:76-66-4
- Bornyl isobutyrate
Catalog No.:BCC8134
CAS No.:50277-27-5
- Oxymorphone
Catalog No.:BCC5255
CAS No.:76-41-5
- Triamcinolone Acetonide
Catalog No.:BCC3871
CAS No.:76-25-5
- Camphor
Catalog No.:BCN8297
CAS No.:76-22-2
- Pancreatic Polypeptide (human)
Catalog No.:BCC5711
CAS No.:75976-10-2
- 11-Hydroxycanthin-6-one
Catalog No.:BCN3104
CAS No.:75969-83-4
- [Nle4,D-Phe7]-α-MSH
Catalog No.:BCC5963
CAS No.:75921-69-6
- Conopharyngine
Catalog No.:BCN3975
CAS No.:76-98-2
- H-DL-Nva-OH
Catalog No.:BCC3303
CAS No.:760-78-1
- Lanatin
Catalog No.:BCC8194
CAS No.:76026-24-9
- 3-Epikatonic acid
Catalog No.:BCN4308
CAS No.:76035-62-6
- Broussonin C
Catalog No.:BCN4588
CAS No.:76045-49-3
- 1-Dehydro-6-gingerdione
Catalog No.:BCN3265
CAS No.:76060-35-0
- Myricoside
Catalog No.:BCC8342
CAS No.:76076-04-5
- Artocarpin
Catalog No.:BCN4309
CAS No.:7608-44-8
- SCH 28080
Catalog No.:BCC7154
CAS No.:76081-98-6
- Enalapril maleate
Catalog No.:BCC8955
CAS No.:76095-16-4
- Hederacoside D
Catalog No.:BCN2330
CAS No.:760961-03-3
- Solithromycin
Catalog No.:BCC6446
CAS No.:760981-83-7
Superiority of pulmonary administration of mepenzolate bromide over other routes as treatment for chronic obstructive pulmonary disease.[Pubmed:24676126]
Sci Rep. 2014 Mar 28;4:4510.
We recently proposed that Mepenzolate Bromide (mepenzolate) would be therapeutically effective against chronic obstructive pulmonary disease (COPD) due to its both anti-inflammatory and bronchodilatory activities. In this study, we examined the benefits and adverse effects associated with different routes of mepenzolate administration in mice. Oral administration of mepenzolate caused not only bronchodilation but also decreased the severity of elastase-induced pulmonary emphysema; however, compared with the intratracheal route of administration, about 5000 times higher dose was required to achieve this effect. Intravenously or intrarectally administered mepenzolate also showed these pharmacological effects. The intratracheal route of mepenzolate administration, but not other routes, resulted in protective effects against elastase-induced pulmonary damage and bronchodilation at a much lower dose than that which affected defecation and heart rate. These results suggest that the pulmonary route of mepenzolate administration may be superior to other routes (oral, intravenous or intrarectal) to treat COPD patients.
Ameliorative effect of mepenzolate bromide against pulmonary fibrosis.[Pubmed:24769542]
J Pharmacol Exp Ther. 2014 Jul;350(1):79-88.
Idiopathic pulmonary fibrosis is thought to involve lung injury caused by reactive oxygen species (ROS), which in turn is followed by abnormal fibrosis. A transforming growth factor (TGF)-beta1-induced increase in myofibroblast number plays an important role in this abnormal fibrosis. We recently found that Mepenzolate Bromide (mepenzolate), which has been used clinically to treat gastrointestinal disorders, has ROS-reducing properties. In the present study, we examined the effect of mepenzolate on bleomycin-induced pulmonary fibrosis and lung dysfunction in mice. The severity of pulmonary fibrosis was assessed by histopathologic evaluation and determination of hydroxyproline levels. Lung mechanics (elastance) and respiratory function [forced vital capacity (FVC)] were assessed using a computer-controlled ventilator. Respiratory function was also evaluated by monitoring percutaneous arterial oxygen saturation (SpO2). Intratracheal administration of mepenzolate prior to bleomycin treatment reduced the extent of pulmonary fibrosis and changes in lung mechanics and led to a significant recovery of both FVC and SpO2 compared with control. Furthermore, mepenzolate produced a therapeutic effect even when it was administered after the development of fibrosis. Administration of mepenzolate also prevented bleomycin-induced pulmonary cell death and inflammatory responses and increased myofibroblast number. Mepenzolate also decreased NADPH oxidase activity and active TGF-beta1 level or increased glutathione S-transferase (GST) activity in the presence of bleomycin treatment. These results show that the intratracheal administration of mepenzolate reduced bleomycin-induced pulmonary fibrosis and lung dysfunction in mice. These effects may be due to this drug's inhibitory effect on NADPH oxidase and TGF-beta1 activities and its stimulatory effect on GST.
Mepenzolate bromide promotes diabetic wound healing by modulating inflammation and oxidative stress.[Pubmed:27398156]
Am J Transl Res. 2016 Jun 15;8(6):2738-47. eCollection 2016.
Diabetic wounds are characterized by persistent inflammation and the excessive production of reactive oxygen species, thus resulting in impaired wound healing. Mepenzolate Bromide, which was originally used to treat gastrointestinal disorders in clinical settings, has recently been shown to display beneficial effects in chronic obstructive pulmonary disease and pulmonary fibrosis of a mouse model by inhibiting inflammatory responses and reducing oxidative stress. However,the role of Mepenzolate Bromide in diabetic wound healing is still unclear. In this study, full-thickness excisional skin wounds were created on the backs of db/db mice, and Mepenzolate Bromide was topically applied to the wound bed. We found that Mepenzolate Bromide significantly promoted diabetic wound healing by measuring wound closure rate and histomorphometric analyses. Further studies showed that inflammation was inhibited by assessing the number of macrophages and levels of pro-inflammatory cytokines and pro-healing cytokines in the wounds. Furthermore, oxidative stress was reduced by monitoring the levels of MDA and H2O2 and the activities of glutathione peroxidase and catalase in the wounds. These results demonstrated the potential application of Mepenzolate Bromide for treating diabetic ulcers and other chronic wounds in clinics.
Synthesis and biological comparison of enantiomers of mepenzolate bromide, a muscarinic receptor antagonist with bronchodilatory and anti-inflammatory activities.[Pubmed:24844758]
Bioorg Med Chem. 2014 Jul 1;22(13):3488-97.
Chronic obstructive pulmonary disease (COPD) is characterized by abnormal inflammatory responses and airflow limitations. We recently proposed that the muscarinic antagonist Mepenzolate Bromide (mepenzolate) would be therapeutically effective against COPD due to its muscarinic receptor-dependent bronchodilatory activity as well as anti-inflammatory properties. Mepenzolate has an asymmetric carbon atom, thus providing us with the opportunity to synthesize both of its enantiomers ((R)- and (S)-mepenzolate) and to examine their biochemical and pharmacological activities. (R)- or (S)-mepenzolate was synthesized by condensation of benzilic acid with (R)- or (S)-alcohol, respectively, followed by quaternization of the tertiary amine. As predicted by computational simulation, a filter-binding assay in vitro revealed that (R)-mepenzolate showed a higher affinity for the muscarinic M3 receptor than (S)-mepenzolate. In vivo, the bronchodilatory activity of (R)-mepenzolate was superior to that of (S)-mepenzolate, whereas anti-inflammatory activity was indistinguishable between the two enantiomers. We confirmed that each mepenzolate maintained its original stereochemistry in the lung when administered intratracheally. These results suggest that (R)-mepenzolate may have superior properties to (S)-mepenzolate as a drug to treat COPD patients given that the former has more potent bronchodilatory activity than the latter.


