PrilocaineCAS# 721-50-6 |
- Istradefylline (KW-6002)
Catalog No.:BCC3798
CAS No.:155270-99-8
- ANR 94
Catalog No.:BCC7815
CAS No.:634924-89-3
- Tozadenant
Catalog No.:BCC2011
CAS No.:870070-55-6
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 721-50-6 | SDF | Download SDF |
| PubChem ID | 4906 | Appearance | Powder |
| Formula | C13H20N2O | M.Wt | 220.31 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble in DMSO > 10 mM | ||
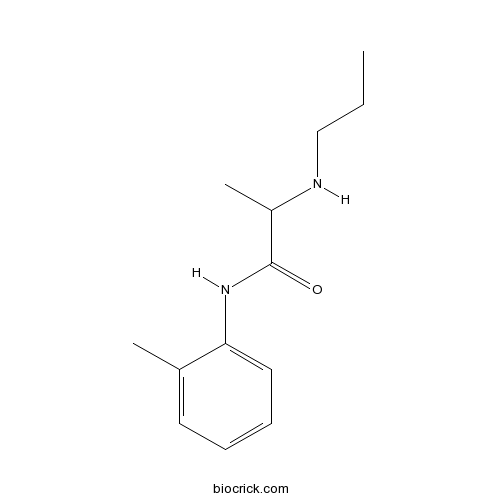
| Chemical Name | N-(2-methylphenyl)-2-(propylamino)propanamide | ||
| SMILES | CCCNC(C)C(=O)NC1=CC=CC=C1C | ||
| Standard InChIKey | MVFGUOIZUNYYSO-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C13H20N2O/c1-4-9-14-11(3)13(16)15-12-8-6-5-7-10(12)2/h5-8,11,14H,4,9H2,1-3H3,(H,15,16) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Prilocaine is a local anesthetic of the amino amide type.
Target: Others
Prilocaine is a local anesthetic of the amino amide type first prepared by Claes Tegner and Nils L?fgren. In its injectable form (trade name Citanest), it is often used in dentistry. It is also often combined with lidocaine as a preparation for dermal anesthesia, for treatment of conditions like paresthesia. As it has low cardiac toxicity, it is commonly used for intravenous regional anaesthesia (IVRA). In some patients, a metabolite of prilocaine may cause the unusual side effect of methemoglobinemia, which may be treated with methylene blue. Maximum dosage for dental use: 8.0 mg/kg (2.7 mg/lb), with a maximum dose of 500 mg.
Eutectic Mixture of Local Anesthetics (EMLA) containing 5% lidocaine and prilocaine in a cream was found to give effective topical analgesia in normal and diseased skin, making it useful for superficial surgery and various other clinical procedures. To be effective, an adequate amount must be applied under occlusion and at the right time before the intervention. References: | |||||

Prilocaine Dilution Calculator

Prilocaine Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.5391 mL | 22.6953 mL | 45.3906 mL | 90.7812 mL | 113.4765 mL |
| 5 mM | 0.9078 mL | 4.5391 mL | 9.0781 mL | 18.1562 mL | 22.6953 mL |
| 10 mM | 0.4539 mL | 2.2695 mL | 4.5391 mL | 9.0781 mL | 11.3476 mL |
| 50 mM | 0.0908 mL | 0.4539 mL | 0.9078 mL | 1.8156 mL | 2.2695 mL |
| 100 mM | 0.0454 mL | 0.227 mL | 0.4539 mL | 0.9078 mL | 1.1348 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
Prilocaine is a local anesthetic of the amino amide type.
- Mastoparan X
Catalog No.:BCC5833
CAS No.:72093-22-2
- 5(6)-Carboxyfluorescein
Catalog No.:BCC8283
CAS No.:72088-94-9
- 6,2',4'-Trimethoxyflavone
Catalog No.:BCC3897
CAS No.:720675-74-1
- Spinosin
Catalog No.:BCN1644
CAS No.:72063-39-9
- Sepinol
Catalog No.:BCN4277
CAS No.:72061-63-3
- Lochnericine
Catalog No.:BCN4595
CAS No.:72058-36-7
- NHS-LC-Biotin
Catalog No.:BCC3579
CAS No.:72040-63-2
- Henryoside
Catalog No.:BCN4276
CAS No.:72021-23-9
- 2-Geranyl-4-isobutyrylphloroglucinol
Catalog No.:BCN7170
CAS No.:72008-03-8
- Chlorquinaldol
Catalog No.:BCC4648
CAS No.:72-80-0
- Metandienone
Catalog No.:BCC9025
CAS No.:72-63-9
- 2,2-Bis(4-chlorophenyl)-1,1-dichloroethylene
Catalog No.:BCC8493
CAS No.:72-55-9
- Nerolidol
Catalog No.:BCN5459
CAS No.:7212-44-4
- b-Casomorphin (1-3)
Catalog No.:BCC1007
CAS No.:72122-59-9
- 2-Hydroxyplatyphyllide
Catalog No.:BCN7119
CAS No.:72145-19-8
- Aflatoxin B2
Catalog No.:BCC9213
CAS No.:7220-81-7
- Calcitetrol
Catalog No.:BCC1446
CAS No.:72203-93-1
- Parsonsine
Catalog No.:BCN2111
CAS No.:72213-98-0
- Echitovenidine
Catalog No.:BCN7482
CAS No.:7222-35-7
- 2-Hydroxyeupatolide
Catalog No.:BCN2490
CAS No.:72229-33-5
- 2alpha-Hydroxyeupatolide 8-O-angelate
Catalog No.:BCN7340
CAS No.:72229-39-1
- Laurifoline
Catalog No.:BCN4278
CAS No.:7224-61-5
- Org 24598 lithium salt
Catalog No.:BCC7845
CAS No.:722456-08-8
- AZD1152
Catalog No.:BCC1393
CAS No.:722543-31-9
First-time success with needle procedures was higher with a warm lidocaine and tetracaine patch than an eutectic mixture of lidocaine and prilocaine cream.[Pubmed:28130888]
Acta Paediatr. 2017 May;106(5):773-778.
AIM: More than 50% of children report apian during venepuncture or intravenous cannulation and using local anaesthetics before needle procedures can lead to different success rates. This study examined how many needle procedures were successful at the first attempt when children received either a warm lidocaine and tetracaine patch or an eutectic mixture of lidocaine and Prilocaine (EMLA) cream. METHODS: We conducted this multicentre randomised controlled trial at three tertiary-level children's hospitals in Italy in 2015. Children aged three to 10 years were enrolled in an emergency department, paediatric day hospital and paediatric ward and randomly allocated to receive a warm lidocaine and tetracaine patch or EMLA cream. The primary outcome was the success rate at the first attempt. RESULTS: The analysis included 172 children who received a warm lidocaine and tetracaine patch and 167 who received an EMLA cream. The needle procedure was successful at the first attempt in 158 children (92.4%) who received the warm patch and in 142 children (85.0%) who received the cream (p = 0.03). The pain scores were similar in both groups. CONCLUSION: This study showed that the first-time needle procedure success was 7.4% higher in children receiving a warm lidocaine and tetracaine patch than EMLA cream.
Effect of cervical lidocaine-prilocaine cream on pain perception during copper T380A intrauterine device insertion among parous women: A randomized double-blind controlled trial.[Pubmed:27823944]
Contraception. 2017 Mar;95(3):251-256.
OBJECTIVE: The objective was to investigate the analgesic effect of cervical lidocaine-Prilocaine (LP) cream in alleviating pain during copper T380A intrauterine device (IUD) insertion among parous women. STUDY DESIGN: We conducted a randomized, double-blind, placebo-controlled trial at Assiut Women's Health Hospital, Egypt, from October 2015 to April 2016 of parous women desiring copper IUD insertion. We randomized the subjects in a 1:1 ratio to LP cream or placebo. Seven minutes prior to IUD insertion, women received 2 ml of LP cream or placebo to the anterior cervical lip, followed by 2 ml placed in the cervical canal using a Q-tip applicator. The study end point was the subjects' self-reported pain using a 10-cm visual analog scale (VAS) during cervical tenaculum placement, sound insertion, IUD insertion and 5 min postprocedure. We considered a 2-cm difference in VAS scores between study groups as clinically significant. Also, the difference in the ease of insertion score using a 10-cm VAS with 0=very easy insertion and 10=terribly difficult insertion was assessed. RESULTS: The study included 120 women (n=60 in each group). LP cream reduces the median VAS pain scores during tenaculum placement (2 vs. 4), sound insertion (3 vs. 6) and IUD insertion (3 vs. 6.5) with p=.0001 at all steps. A lower ease of insertion score was also determined among LP women (2.5+/-0.98 vs. 4.5+/-2.7, p=.001). Participants reported no side effects. CONCLUSIONS: Use of cervical LP cream prior to copper T380A IUD insertion may alleviate the IUD insertion pain among parous women. IMPLICATIONS: Cervical LP cream could be effective as an analgesic prior to copper T380A IUD insertion with no side effects. Further studies are needed to assess the women's satisfaction from lying with a speculum in place for 7 min while waiting for the cream to be effective.
The effect of a lidocaine/prilocaine topical anesthetic on pain and discomfort associated with orthodontic elastomeric separator placement.[Pubmed:28066874]
Prog Orthod. 2017 Dec;18(1):1.
BACKGROUND: The initial placement of orthodontic elastomeric separators can be uncomfortable and painful. Therefore, it is important to relieve this disturbing sensation to create a discomfort or pain-free orthodontic visit. The purpose of this study was to investigate the effect of a lidocaine/Prilocaine topical anesthetic on pain and discomfort associated with the placement of orthodontic elastomeric separators. METHODS: Fifty subjects aging between 20-35 years were included in this study. In the maxillary arch, a lidocaine/Prilocaine topical anesthetic was placed around the ginigval margins of the premolar and molar on side. On the other side, a placebo agent was placed around the ginigval margins of the premolar and molar. After two minutes, an elastomeric separator was placed between the premolar and molar on both sides. The subjects were then asked to report their findings on a Verbal Scale and a Visual Analogue Scale every second minute for a period of 10 min. The subjects were also given a questionnaire to evaluate the overall impression on the topical anesthetic use. RESULTS: The overall mean discomfort/pain score was found to be significantly lower (p < 0.001) with the topical anesthetic than with the placebo. Repeated measures ANOVA with a Greenhouse-Geisser correction determined that mean pain scores were statistically significantly low with the 10-min time duration (F (1.54,42.2) = 40.7, p = 0.001), with an estimated grand mean (8.37, 95% CI 6.75-9.98). The questionnaire responses revealed that 87% of the subjects reported an overall satisfaction and agreement with the topical anesthetic than with the placebo or no difference (13%) after the initial separator placement. CONCLUSIONS: The discomfort and pain resulting from the initial placement of orthodontic elastomeric separators can be significantly reduced with the lidocaine/Prilocaine topical anesthetic.
A double-blind randomized prospective study comparing prilocaine versus ropivacaine in upper blepharoplasty.[Pubmed:28017546]
J Plast Reconstr Aesthet Surg. 2017 Mar;70(3):375-379.
BACKGROUND: Upper blepharoplasties are a common procedure in plastic surgery. This procedure can be performed effectively under local anesthesia with or without sedation. The ideal local anesthetic should cause less intraoperative bleeding and less postoperative edema. Our study aimed to show the difference between the two local anesthetics 1% Prilocaine (Xylonaest) in combination with epinephrine 1:100,000 and ropivacaine (Naropin) in combination with epinephrine 1:100,000 including sodium chloride, particularly in regard to swelling and bleeding in patients undergoing upper blepharoplasties. MATERIAL AND METHODS: In this double-blind, prospective, randomized study, 31 patients between March 2014 and September 2014 were included. The anesthetic agents used in all cases were 1% Prilocaine (Xylonaest) in combination with epinephrine 1:100,000 for one side and ropivacaine consisting of 10-mg Naropin, 5-ml sodium chloride, and 1-ml epinephrine for the other side. The data presented in this study were collected by one of the surgeons performing the surgery. Intraoperative bleeding and postoperative edema were both calculated using a score of five points for each. RESULTS: The average bleeding tendency was 3.39 for Prilocaine and 1.71 for local ropivacaine, showing a significant difference (p < 0.0001) between both local anesthetics in bleeding tendency. There was also a significant minor swelling at all times on the side on which ropivacaine was used. DISCUSSION: In our study, we demonstrated that ropivacaine (Naropin) has less intra- and postoperative side effects including swelling and bleeding compared with Prilocaine (Xylonaest).


