FGIN-1-27Potent, specific ligand for mitochondrial DBI receptor CAS# 142720-24-9 |
- BAY 87-2243
Catalog No.:BCC4131
CAS No.:1227158-85-1
- PX 12
Catalog No.:BCC2436
CAS No.:141400-58-0
- DMOG
Catalog No.:BCC2433
CAS No.:89464-63-1
- KC7F2
Catalog No.:BCC2434
CAS No.:927822-86-4
- IOX2(Glycine)
Catalog No.:BCC2229
CAS No.:931398-72-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 142720-24-9 | SDF | Download SDF |
| PubChem ID | 132496 | Appearance | Powder |
| Formula | C28H37FN2O | M.Wt | 436.61 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO | ||
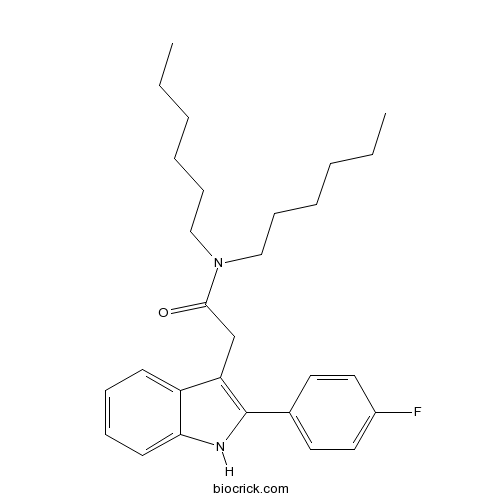
| Chemical Name | 2-[2-(4-fluorophenyl)-1H-indol-3-yl]-N,N-dihexylacetamide | ||
| SMILES | CCCCCCN(CCCCCC)C(=O)CC1=C(NC2=CC=CC=C21)C3=CC=C(C=C3)F | ||
| Standard InChIKey | VUWXAQFLTSBUDB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C28H37FN2O/c1-3-5-7-11-19-31(20-12-8-6-4-2)27(32)21-25-24-13-9-10-14-26(24)30-28(25)22-15-17-23(29)18-16-22/h9-10,13-18,30H,3-8,11-12,19-21H2,1-2H3 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Binds specifically and with high affinity to the peptide DBI receptor (benzodiazepine receptor) on mitochondrial membranes, inducing production of neurosteroids, which modulate GABA receptors (EC50 = 3 nM for increase in production of pregnenolone in glial cells). Thus it reduces anxiety without causing sedation. |

FGIN-1-27 Dilution Calculator

FGIN-1-27 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2904 mL | 11.4519 mL | 22.9037 mL | 45.8075 mL | 57.2593 mL |
| 5 mM | 0.4581 mL | 2.2904 mL | 4.5807 mL | 9.1615 mL | 11.4519 mL |
| 10 mM | 0.229 mL | 1.1452 mL | 2.2904 mL | 4.5807 mL | 5.7259 mL |
| 50 mM | 0.0458 mL | 0.229 mL | 0.4581 mL | 0.9161 mL | 1.1452 mL |
| 100 mM | 0.0229 mL | 0.1145 mL | 0.229 mL | 0.4581 mL | 0.5726 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Dihydrocurcumenone
Catalog No.:BCN3557
CAS No.:142717-57-5
- CP 100356 hydrochloride
Catalog No.:BCC7882
CAS No.:142715-48-8
- Macrocarpal B
Catalog No.:BCN6236
CAS No.:142698-60-0
- Genkwanol B
Catalog No.:BCN8013
CAS No.:142674-67-7
- 6-Hydroxykaempferol 3,6-diglucoside
Catalog No.:BCN3335
CAS No.:142674-16-6
- Macrocarpal D
Catalog No.:BCN6235
CAS No.:142647-71-0
- Macrocarpal E
Catalog No.:BCN6234
CAS No.:142628-54-4
- Macrocarpal C
Catalog No.:BCN6233
CAS No.:142628-53-3
- Daphylloside
Catalog No.:BCN6232
CAS No.:14260-99-2
- Deacetylasperulosidic acid
Catalog No.:BCN3323
CAS No.:14259-55-3
- Didymin
Catalog No.:BCN3327
CAS No.:14259-47-3
- Narirutin
Catalog No.:BCN6300
CAS No.:14259-46-2
- Conophylline
Catalog No.:BCN6237
CAS No.:142741-24-0
- Buddlenoid A
Catalog No.:BCN8210
CAS No.:142750-32-1
- Anemarsaponin BIII
Catalog No.:BCN2898
CAS No.:142759-74-8
- 12-Hydroxysapriparaquinone
Catalog No.:BCN3216
CAS No.:142763-37-9
- Domoic acid
Catalog No.:BCC6586
CAS No.:14277-97-5
- IWP-L6
Catalog No.:BCC5101
CAS No.:1427782-89-5
- 4-Chlorophenylguanidine hydrochloride
Catalog No.:BCC2382
CAS No.:14279-91-5
- Silybin B
Catalog No.:BCN7898
CAS No.:142797-34-0
- 5,7-Dimethoxyluteolin
Catalog No.:BCN8167
CAS No.:90363-40-9
- DPN
Catalog No.:BCC7088
CAS No.:1428-67-7
- Apiodionene
Catalog No.:BCN1829
CAS No.:142808-38-6
- Preapiodionene
Catalog No.:BCN1854
CAS No.:142808-39-7
In the ventral tegmental area picrotoxin blocks FGIN 1-27-induced increases in sexual behavior of rats and hamsters.[Pubmed:15338106]
Psychopharmacology (Berl). 2005 Mar;178(2-3):174-82.
RATIONALE AND OBJECTIVES: There are two types of benzodiazepine receptors, mitochondrial benzodiazepine receptors (MBRs), and gamma-aminobutyric acid (GABA(A))/benzodiazepine receptor complexes (GBRs). MBR activation increases neurosteroidogenesis. Ventral tegmental area (VTA) infusions of the MBR agonist, FGIN 1-27, increase midbrain levels of the progesterone metabolite 5alpha-pregnan-3alpha-ol-20-one (3alpha,5alpha-THP) and lordosis of rats and hamsters. Activation of GBRs leads to membrane hyperpolarization. In the VTA, infusions of GBR agonists enhance 3alpha,5alpha-THP-facilitated lordosis. Thus, if, in the VTA, MBR-mediated increases in 3alpha,5alpha-THP enhance sexual responses via actions at GBRs, then blocking GBRs with picrotoxin will reduce FGIN 1-27-induced increases in sexual behavior of female rodents. METHODS: Ovariectomized rats and hamsters, with unilateral guide cannula to the VTA, received estradiol benzoate (10 mug; EB) at hour 0. Hamsters also received progesterone (100 mug) at hour 44. At hour 47.5, all animals were infused first with 10 ng or 20 ng picrotoxin or saline, vehicle to the VTA and, 30 min later, with 5 mug/11.4 nM FGIN 1-27 or saline, vehicle. Ten minutes later, animals were tested for sex and motor behavior. RESULTS: Picrotoxin, but not vehicle, infusions blocked FGIN 1-27-mediated increases in lordosis of rats and hamsters, proceptivity of rats, and sexual responsiveness of hamsters. In addition, midbrain 3alpha,5alpha-THP levels were higher in animals that received VTA infusions of FGIN 1-27, compared to those infused with saline, vehicle. CONCLUSION: In the VTA, GBRs are required for MBR-enhanced sexual behavior of EB-primed rats and EB- and progesterone-primed hamsters.
Preparation of indoles from alpha-aminonitriles: A short synthesis of FGIN-1-27.[Pubmed:16986928]
Org Lett. 2006 Sep 28;8(20):4473-5.
Alpha-Aminonitriles derived from 2-aminocinnamic acid esters and amides can be cyclized under basic conditions to furnish substituted indole-3-acetic acid derivatives in quantitative yield. The reaction provides a simple access to a class of biologically active compounds.
In vitro effect of FGIN-1-27, a ligand to 18 kDa mitochondrial translocator protein, in human osteoblast-like cells.[Pubmed:24532136]
J Bioenerg Biomembr. 2014 Jun;46(3):197-204.
Ligands of 18 kDa mitochondrial translocator protein (TSPO) differ in their cellular effects. We hypothesize that different TSPO ligands might exert different cellular responses. Therefore, following previous studies that showed different cellular responses to two specific TSPO ligands, PK 11195 and protoporphyrin IX, in human osteoblast-like cells in vitro, we now report the cellular response to another specific TSPO ligand, FGIN-1-27 (10(-5) M) (MW 436 kDa), in order to characterize the effects of each TSPO ligand. We found in primary culture of the human osteoblast-like cells that cell numbers were decreased by an average of 30% (p < 0.001) following exposure to 10(-5) M of FGIN-1-27 in comparison to vehicle controls. Cellular [(18)F]-FDG incorporation and ATP content were suppressed, by an average of 43% (p < 0.001) and 83% (p < 0.001), respectively. Mitochondrial mass and DeltaPsim increased by an average of 26% (p < 0.01) and 425% (p < 0.0001) respectively. Lactate dehydrogenase activity was enhanced in culture media by 60% (p < 0.05), indicating overall cell death, while no increase in apoptotic levels was observed. Cellular proliferation, as determined by BrdU assay, was not affected. Synthesis of mRNA of TSPO, VDAC 1, and hexokinase 2 decreased in 0.3, 0.3 and 0.5 fold respectively, with accompanying decreases in protein expression of TSPO and Voltage Dependent Anion Channel 1 by 23% (p < 0.001) and 98% (p < 0.001), respectively, but without changes in hexokinase 2 protein expression. Thus it appears that 10(-5) M FGIN-1-27 reduces cell viability, cell metabolism, and mitochondrial function. Previously we found similar effects of PK 11195 on mitochondrial function and cell metabolism and of protoporphyrin IX on cell death in primary osteoblast-like cells.
Synthesis and preliminary behavioural evaluation in mice of new 3-aryl-3-pyrrol-1-ylpropanamides, analogues of FGIN-1-27 and FGIN-1-43.[Pubmed:11732760]
J Pharm Pharmacol. 2001 Nov;53(11):1561-8.
The 2-aryl-3-indoleacetamides FGIN-1-27 and FGIN-1-43 have already been characterized in-vitro as potent and specific ligands for the mitochondrial DBI receptor. This affinity was associated with psychotropic properties in several rodent behavioural tasks (in particular anxiolytic action) via enhancement of GABA transmission through neurosteroid production. The synthesis of new 3-aryl-3-pyrrol-1-ylpropanamides 1a-i, analogues of FGIN-1-27 and FGIN-1-43, is described in four steps starting from the corresponding arylaldehydes. Preliminary evaluation of these compounds in behavioural studies (spontaneous locomotor activity and anxiolytic activity) in mice was also undertaken.
2-Aryl-3-indoleacetamides (FGIN-1): a new class of potent and specific ligands for the mitochondrial DBI receptor (MDR).[Pubmed:1326631]
J Pharmacol Exp Ther. 1992 Sep;262(3):971-8.
The 2 aryl-3-indoleacetamides (FGIN-1) are a new class of compounds that potently (nM) and selectively bind to glial mitochondrial diazepam binding inhibitor (DBI) receptors (MDR), previously called peripheral benzodiazepine receptors, and increase mitochondrial steroidogenesis. The high-affinity binding of FGIN-1 to MDR derivatives depends on the following chemical characteristics: 1) the dialkylation of the amide; 2) the chain length of this alkyl substitution; and 3) the halogenation of aryl groups appended to the indole nucleus. FGIN-1 derivatives do not bind to gamma-aminobutyric acid (GABAA), GABAB, glycine, glutamate, dopamine, serotonin, opiate, cholecystokinin, beta adrenergic, cannabinoid or sigma receptors. FGIN-1-27 [N, N-di-n-hexyl 2-(4-fluorophenyl)indole-3-acetamide] enters the brain, and for this reason, this FGIN-1 compound is potent and efficacious behaviorally. Like the neurosteroid 3 alpha-5 alpha tetrahydrodeoxycorticosterone (THDOC), FGIN-1-27 delays the onset of isoniazid-induced convulsions, but fails to delay the onset of bicuculline-induced convulsions. However, differently from THDOC, the FGIN-1-27 anticonvulsant action is blocked by the isoquinoline carboxamide PK 11195. In the elevated plus maze test, FGIN-1-27 inhibits neophobia manner that is antagonized by PK 11195 but not by flumazenil. Because FGIN-1-27 binds to MDR and does not bind to the GABAA receptors, it is inferred that FGIN-1-27 may act on GABAA receptors indirectly, presumably via a stimulation of neurosteroid synthesis and release from glial cells.


