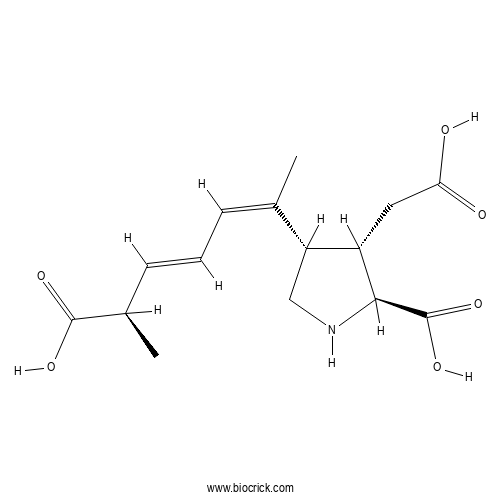
Domoic acidCAS# 14277-97-5 |
- Repaglinide
Catalog No.:BCC2504
CAS No.:135062-02-1
- Dronedarone
Catalog No.:BCN2176
CAS No.:141626-36-0
- NS309
Catalog No.:BCC1809
CAS No.:18711-16-5
- TRAM-34
Catalog No.:BCC1122
CAS No.:289905-88-0
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 14277-97-5 | SDF | Download SDF |
| PubChem ID | 5282253 | Appearance | Powder |
| Formula | C15H21NO6 | M.Wt | 311.33 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Domoate, (−)-Domoic Acid, L-Domoic Acid | ||
| Solubility | Soluble to 50 mM in water | ||
| Chemical Name | (2S,3S,4S)-2-carboxy-4-[(1Z,3E,5R)-5-carboxy-1-methyl-1,3-hexadien-1-yl]-3-pyrrolidineacetic acid | ||
| SMILES | CC(C=CC=C(C)C1CNC(C1CC(=O)O)C(=O)O)C(=O)O | ||
| Standard InChIKey | VZFRNCSOCOPNDB-AOKDLOFSSA-N | ||
| Standard InChI | InChI=1S/C15H21NO6/c1-8(4-3-5-9(2)14(19)20)11-7-16-13(15(21)22)10(11)6-12(17)18/h3-5,9-11,13,16H,6-7H2,1-2H3,(H,17,18)(H,19,20)(H,21,22)/b5-3+,8-4-/t9-,10+,11-,13+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Kainate receptor agonist. More potent and possibly more selective than kainate at kainate receptors, as demonstrated in electrophysiological studies. |

Domoic acid Dilution Calculator

Domoic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.212 mL | 16.0601 mL | 32.1203 mL | 64.2405 mL | 80.3006 mL |
| 5 mM | 0.6424 mL | 3.212 mL | 6.4241 mL | 12.8481 mL | 16.0601 mL |
| 10 mM | 0.3212 mL | 1.606 mL | 3.212 mL | 6.4241 mL | 8.0301 mL |
| 50 mM | 0.0642 mL | 0.3212 mL | 0.6424 mL | 1.2848 mL | 1.606 mL |
| 100 mM | 0.0321 mL | 0.1606 mL | 0.3212 mL | 0.6424 mL | 0.803 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 12-Hydroxysapriparaquinone
Catalog No.:BCN3216
CAS No.:142763-37-9
- Anemarsaponin BIII
Catalog No.:BCN2898
CAS No.:142759-74-8
- Buddlenoid A
Catalog No.:BCN8210
CAS No.:142750-32-1
- Conophylline
Catalog No.:BCN6237
CAS No.:142741-24-0
- FGIN-1-27
Catalog No.:BCC6738
CAS No.:142720-24-9
- Dihydrocurcumenone
Catalog No.:BCN3557
CAS No.:142717-57-5
- CP 100356 hydrochloride
Catalog No.:BCC7882
CAS No.:142715-48-8
- Macrocarpal B
Catalog No.:BCN6236
CAS No.:142698-60-0
- Genkwanol B
Catalog No.:BCN8013
CAS No.:142674-67-7
- 6-Hydroxykaempferol 3,6-diglucoside
Catalog No.:BCN3335
CAS No.:142674-16-6
- Macrocarpal D
Catalog No.:BCN6235
CAS No.:142647-71-0
- Macrocarpal E
Catalog No.:BCN6234
CAS No.:142628-54-4
- IWP-L6
Catalog No.:BCC5101
CAS No.:1427782-89-5
- 4-Chlorophenylguanidine hydrochloride
Catalog No.:BCC2382
CAS No.:14279-91-5
- Silybin B
Catalog No.:BCN7898
CAS No.:142797-34-0
- 5,7-Dimethoxyluteolin
Catalog No.:BCN8167
CAS No.:90363-40-9
- DPN
Catalog No.:BCC7088
CAS No.:1428-67-7
- Apiodionene
Catalog No.:BCN1829
CAS No.:142808-38-6
- Preapiodionene
Catalog No.:BCN1854
CAS No.:142808-39-7
- Clinopodiside A
Catalog No.:BCN2621
CAS No.:142809-89-0
- L-Sulforaphane
Catalog No.:BCN8449
CAS No.:142825-10-3
- Clausine D
Catalog No.:BCN4707
CAS No.:142846-95-5
- N-Acetyl-N-acetoxy-4-chlorobenzenesulfonamide
Catalog No.:BCC6762
CAS No.:142867-52-5
- Toddacoumaquinone
Catalog No.:BCN3640
CAS No.:142878-03-3
Modulatory effects of Terminalia arjuna against domoic acid induced toxicity in Caco-2 cell line.[Pubmed:28342004]
Cytotechnology. 2017 Aug;69(4):725-739.
Domoic acid is a potent marine algal toxin produced by diatomic genus of Pseudo-nitzschia causing amnesic shell fish poisoning. Domoic acid toxicosis mainly involves excitotoxic effects coupled with oxidative stress. The present study was aimed to evaluate the protective effects of hydro-alcoholic extract of Terminalia arjuna (TA) against Domoic acid induced toxic effects in Caco-2 cell line. It was observed that the toxicity induced by Domoic acid in Caco-2 cells was mediated by oxidative insult leading to morphological changes, DNA damage and apoptosis. In our study pre-treatment of the cells with TA (10, 20 and 30 mug/ml) showed significant protection against Domoic acid induced morphological, oxidative and apoptotic damages in a dose dependent manner. The effect of phytocompounds present in TA viz., kaempferol and arjungenin showed significant protection against Domoic acid induced toxicity in Caco-2 cell line. Hence, it could be inferred that the protective effect of TA extract against Domoic acid induced toxicity could be due to the individual or synergistic effects of kaempferol and argungenin. However, further clinical studies are warranted to consider TA as a natural remedy to prevent amnesic shell fish poisoning.
Microsatellite Markers for Population Genetic Applications in the Domoic Acid-producing Diatom Pseudo-nitzschia australis Frenguelli (Bacillariophyceae).[Pubmed:28285260]
Protist. 2017 Apr;168(2):197-205.
Microsatellites are commonly used markers in population genetics and are increasingly being employed to determine population structure in phytoplankton populations. We have developed seven polymorphic microsatellite markers for the domoic-acid producing diatom Pseudo-nitzschia australis. Using these markers, thirty P. australis isolates were genotyped, 10 isolates were from Monterey Bay, California and 20 were from off the northern coast of Oregon. The number of alleles per locus ranged from two to eight and observed heterozygosities ranged from 0.11 to 0.70. All but two of the isolates were genetically distinct and initial population differentiation analysis indicated no significant differences between the Pacific Northwest isolates and the Monterey Bay isolates. Pseudo-nitzschia australis microsatellites appear to be species specific based on cross amplification tests with Pseudo-nitzschia fraudulenta (Cleve) Hasle, Pseudo-nitzschia seriata (Cleve) H.Peragallo, Pseudo-nitzschia pungens (Grunow ex Cleve) and Pseudo-nitzschia multiseries (Hasle) Hasle.
The relationship between Pseudo-nitzschia (Peragallo) and domoic acid in Scottish shellfish.[Pubmed:28366394]
Harmful Algae. 2017 Mar;63:193-202.
The diatom genus Pseudo-nitzschia (Peragallo) associated with the production of Domoic acid (DA), the toxin reposnsible for amnesic shellfish poisoning, is abundant in Scottish waters. A two year study examined the relationship between Pseudo-nitzschia cells in the water column and DA concentration in blue mussels (Mytilus edulis) at two sites, and king scallops (Pecten maximus) at one site. The rate of DA uptake and depuration differed greatly between the two species with M. edulis whole tissue accumulating and depurating 7mugg(-1) (now expressed as mgkg(-1)) per week. In contrast, it took 12 weeks for DA to depurate from P. maximus gonad tissue from a concentration of 68mugg(-1) (now mgkg(-1)) to <20mugg(-1) (now mgkg(-1)). The DA depuration rate from P. maximus whole tissue was <5% per week during both years of the study. Correlations between the Pseudo-nitzschia cell densities and toxin concentrations were weak to moderate for M. edulis and weak for P. maximus. Seasonal diversity on a species level was observed within the Pseudo-nitzschia genus at both sites with more DA toxicity associated with summer/autumn Pseudo-nitzschia blooms when P. australis was observed in phytoplankton samples. This study reveals the marked difference in DA uptake and depuration in two shellfish species of commercial importance in Scotland. The use of these shellfish species to act as a proxy for DA in the environment still requires investigation.
Species occurrence of the potentially toxigenic diatom genus Pseudo-nitzschia and the associated neurotoxin domoic acid in the Argentine Sea.[Pubmed:28366399]
Harmful Algae. 2017 Mar;63:45-55.
The marine diatom genus Pseudo-nitzschia, the major known producer of the neurotoxin Domoic acid (DA) responsible for the amnesic shellfish poisoning (ASP) syndrome in humans and marine mammals, is globally distributed. The genus presents high species richness in the Argentine Sea and DA has been frequently detected in the last few years in plankton and shellfish samples, but the species identity of the producers remains unclear. In the present work, the distribution and abundance of Pseudo-nitzschia species and DA were determined from samples collected on two oceanographic cruises carried out through the Argentine Sea ( approximately 39-47 degrees S) during summer and spring 2013. Phytoplankton composition was analysed by light and electron microscopy while DA was determined by liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS). The genus Pseudo-nitzschia was recorded in 71 and 86% of samples collected in summer and spring, respectively, whereas DA was detected in only 42 and 21% of samples, respectively. Microscopic analyses revealed at least five potentially toxic species (P. australis, P. brasiliana, P. fraudulenta, P. pungens, P. turgidula), plus putatively non-toxigenic P. dolorosa, P. lineola, P. turgiduloides and unidentified specimens of the P. pseudodelicatissima complex. The species P. australis showed the highest correlation with DA occurrence (r=0.55; p<0.05), suggesting its importance as a major DA producer in the Argentine Sea. In the northern area and during summer, DA was associated with the presence of P. brasiliana, a species recorded for the first time in the Argentine Sea. By contrast, high concentrations of P. fraudulenta, P. pungens and P. turgidula did not correspond with DA occurrence. This study represents the first successful attempt to link toxigenicity with Pseudo-nitzschia diversity and cell abundance in field plankton populations in the south-western Atlantic.
The activation of glutamate receptors by kainic acid and domoic acid.[Pubmed:10223631]
Nat Toxins. 1998;6(3-4):153-8.
The neurotoxins kainic acid and Domoic acid are potent agonists at the kainate and alphaamino-5-methyl-3-hydroxyisoxazolone-4-propionate (AMPA) subclasses of ionotropic glutamate receptors. Although it is well established that AMPA receptors mediate fast excitatory synaptic transmission at most excitatory synapses in the central nervous system, the role of the high affinity kainate receptors in synaptic transmission and neurotoxicity is not entirely clear. Kainate and domoate differ from the natural transmitter, L-glutamate, in their mode of activation of glutamate receptors; glutamate elicits rapidly desensitizing responses while the two neurotoxins elicit non-desensitizing or slowly desensitizing responses at AMPA receptors and some kainate receptors. The inability to produce desensitizing currents and the high affinity for AMPA and kainate receptors are undoubtedly important factors in kainate and domoate-mediated neurotoxicity. Mutagenesis studies on cloned glutamate receptors have provided insight into the molecular mechanisms responsible for these unique properties of kainate and domoate.
Domoic acid, the alleged "mussel toxin," might produce its neurotoxic effect through kainate receptor activation: an electrophysiological study in the dorsal hippocampus.[Pubmed:2540893]
Can J Physiol Pharmacol. 1989 Jan;67(1):29-33.
Domoic acid, an excitatory amino acid structurally related to kainate, was recently identified as being presumably responsible for the recent severe intoxication presented by more than 100 people having eaten mussels grown in Prince Edward Island (Canada). The amino acid kainate has been shown to be highly neurotoxic to the hippocampus, which is the most sensitive structure in the central nervous system. The present in vivo electrophysiological studies were undertaken to determine if Domoic acid exerts its neurotoxic effect via kainate receptor activation. Unitary extracellular recordings were obtained from pyramidal neurons of the CA1 and the CA3 regions of the rat dorsal hippocampus. The excitatory effect of Domoic acid applied by microiontophoresis was compared with that of agonists of the three subtypes of glutamatergic receptors: kainate, quisqualate, and N-methyl-D-aspartate. In CA1, the activation induced by Domoic acid was about threefold greater than that induced by kainate; identical concentrations and similar currents were used. In CA3, Domoic acid was also three times more potent than kainate. However, the most striking finding was that Domoic acid, similar to kainate, was more than 20-fold more potent in the CA3 than in the CA1 region, whereas no such regional difference could be detected with quisqualate and N-methyl-D-aspartate. As the differential regional response of CA1 and CA3 pyramidal neurons to kainate is attributable to the extremely high density of kainate receptors in the CA3 region, these results provide the first electrophysiological evidence that Domoic acid may produce its neurotoxic effects through kainate receptor activation.
The primary afferent depolarizing action of kainate in the rat.[Pubmed:3513882]
Br J Pharmacol. 1986 Feb;87(2):345-55.
Dorsal roots (L3-L7) isolated from immature (1-9 day old) rats were depolarized selectively by kainate (1-100 microM). L-Glutamate (25-100 microM), but not L-aspartate, mimicked the action of kainate. N-methylaspartate had no activity on these preparations and quisqualate was thirty times less active than kainate. Depolarizations evoked by L-glutamate (100-1000 microM) faded rapidly in the presence of L-glutamate. Depolarizations evoked by kainate were depressed during the fade induced by L-glutamate. Certain electrically evoked C-fibre volleys in dorsal roots or leg nerves of rats at any age were selectively depressed or abolished in the presence of kainate. The effect of kainate was more selective than that of gamma-aminobutyric acid or capsaicin. Prolonged treatment of dorsal roots with kainate did not appear to be deleterious to C-fibres. It is suggested that certain primary afferent C-fibres possess kainate receptors which may be activated physiologically by L-glutamate released at their central terminations.


