DPNCAS# 1428-67-7 |
- Imatinib Mesylate (STI571)
Catalog No.:BCC1115
CAS No.:220127-57-1
- Dasatinib (BMS-354825)
Catalog No.:BCC1281
CAS No.:302962-49-8
- Saracatinib (AZD0530)
Catalog No.:BCC1166
CAS No.:379231-04-6
- Nilotinib(AMN-107)
Catalog No.:BCC3643
CAS No.:641571-10-0
- Ponatinib (AP24534)
Catalog No.:BCC2522
CAS No.:943319-70-8
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1428-67-7 | SDF | Download SDF |
| PubChem ID | 102614 | Appearance | Powder |
| Formula | C15H13NO2 | M.Wt | 239.27 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | Diarylpropionitrile | ||
| Solubility | DMSO : 100 mg/mL (417.94 mM; Need ultrasonic) H2O : < 0.1 mg/mL (insoluble) | ||
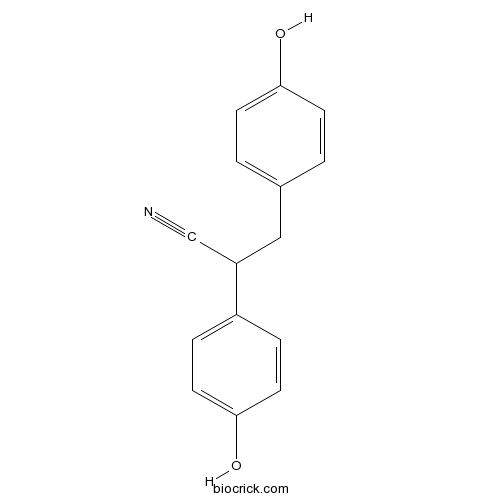
| Chemical Name | 2,3-bis(4-hydroxyphenyl)propanenitrile | ||
| SMILES | C1=CC(=CC=C1CC(C#N)C2=CC=C(C=C2)O)O | ||
| Standard InChIKey | GHZHWDWADLAOIQ-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C15H13NO2/c16-10-13(12-3-7-15(18)8-4-12)9-11-1-5-14(17)6-2-11/h1-8,13,17-18H,9H2 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Highly potent estrogen ERβ receptor agonist with a 70-fold selectivity over ERα (EC50 values are 0.85 and 66 nM respectively). Relaxes mesenteric arteries in vitro. Shown to regulate expression of GluR1, GluR2 and GluR3 in rat hippocampus. |

DPN Dilution Calculator

DPN Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 4.1794 mL | 20.8969 mL | 41.7938 mL | 83.5876 mL | 104.4845 mL |
| 5 mM | 0.8359 mL | 4.1794 mL | 8.3588 mL | 16.7175 mL | 20.8969 mL |
| 10 mM | 0.4179 mL | 2.0897 mL | 4.1794 mL | 8.3588 mL | 10.4484 mL |
| 50 mM | 0.0836 mL | 0.4179 mL | 0.8359 mL | 1.6718 mL | 2.0897 mL |
| 100 mM | 0.0418 mL | 0.209 mL | 0.4179 mL | 0.8359 mL | 1.0448 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 5,7-Dimethoxyluteolin
Catalog No.:BCN8167
CAS No.:90363-40-9
- Silybin B
Catalog No.:BCN7898
CAS No.:142797-34-0
- 4-Chlorophenylguanidine hydrochloride
Catalog No.:BCC2382
CAS No.:14279-91-5
- IWP-L6
Catalog No.:BCC5101
CAS No.:1427782-89-5
- Domoic acid
Catalog No.:BCC6586
CAS No.:14277-97-5
- 12-Hydroxysapriparaquinone
Catalog No.:BCN3216
CAS No.:142763-37-9
- Anemarsaponin BIII
Catalog No.:BCN2898
CAS No.:142759-74-8
- Buddlenoid A
Catalog No.:BCN8210
CAS No.:142750-32-1
- Conophylline
Catalog No.:BCN6237
CAS No.:142741-24-0
- FGIN-1-27
Catalog No.:BCC6738
CAS No.:142720-24-9
- Dihydrocurcumenone
Catalog No.:BCN3557
CAS No.:142717-57-5
- CP 100356 hydrochloride
Catalog No.:BCC7882
CAS No.:142715-48-8
- Apiodionene
Catalog No.:BCN1829
CAS No.:142808-38-6
- Preapiodionene
Catalog No.:BCN1854
CAS No.:142808-39-7
- Clinopodiside A
Catalog No.:BCN2621
CAS No.:142809-89-0
- L-Sulforaphane
Catalog No.:BCN8449
CAS No.:142825-10-3
- Clausine D
Catalog No.:BCN4707
CAS No.:142846-95-5
- N-Acetyl-N-acetoxy-4-chlorobenzenesulfonamide
Catalog No.:BCC6762
CAS No.:142867-52-5
- Toddacoumaquinone
Catalog No.:BCN3640
CAS No.:142878-03-3
- U 73343
Catalog No.:BCC8091
CAS No.:142878-12-4
- GM 6001
Catalog No.:BCC2119
CAS No.:142880-36-2
- 1-O-Ethylpiptocarphin F
Catalog No.:BCN6448
CAS No.:142891-12-1
- 8alpha-Methacryloyloxy-13-ethoxyvernojalcanolide
Catalog No.:BCN7445
CAS No.:142891-14-3
- Petunidin chloride
Catalog No.:BCN3018
CAS No.:1429-30-7
Opioid use in the management of diabetic peripheral neuropathy (DPN) in a large commercially insured population.[Pubmed:25853725]
Clin J Pain. 2015 May;31(5):414-24.
OBJECTIVE: To examine the proportion of diabetic peripheral neuropathy (DPN) patients receiving pharmacologic DPN treatments and specifically to identify the rates and factors associated with opioid use and first-line opioid use. METHODS: A 10% sample of IMS-LifeLink claims data from 1998 through 2008 was used. The study population consisted of diabetic patients who met DPN criteria using a validated DPN algorithm. Multivariable logistic regression controlling for demographics, comorbidities, and other clinical characteristics was used to identify factors associated with any DPN pharmacologic treatment, any opioid use, and first-line opioid treatment. Sensitivity analyses were conducted to explore variations in exclusion criteria as well as opioid use definitions. RESULTS: A total of 666 DPN patients met inclusion criteria and pharmacologic treatment was received by 288 patients (43.24%) and of those, 154 (53.47%) had DPN-related opioid use and 96 (33.33%) received opioid as first-line treatment. Persons with diabetic complications were more likely to use opioids (odds ratio=4.53; 95% confidence interval, 1.09-18.92). Food and Drug Administration-approved DPN agents duloxetine 1.04% (n=3) and pregabalin 5.56% (n=16) had much lower rates of use. DPN-related drug use and DPN-related opioid usage increased as we used less restrictive samples in sensitivity analyses. CONCLUSIONS: Opioids were the most frequently prescribed first-line agents for DPN. More than 50% of DPN patients remained untreated with pharmacologic agents 1 year after a DPN diagnosis.
Improved neural microcirculation and regeneration after peripheral nerve decompression in DPN rats.[Pubmed:28290778]
Neurol Res. 2017 Apr;39(4):285-291.
OBJECTIVE: Recently, neural microcirculation and regeneration were regarded as critical factors in diabetic peripheral neuropathy (DPN) improvement. In the present study, we explored the cytological and molecular mechanisms how peripheral nerve decompression impaired nerve injury. METHODS: Forty-five male SD rats were established as the DPN model. HE staining was used to observe the morphology and distribution of microvessels. Transmission electron microscopy was applied to observe the morphology and distribution of Schwann cells. Immunohistochemical staining was performed to measure nerve growth factor (NGF), tyrosine kinase receptor A (TrkA) and growth-associated protein 43 (GAP-43) in the distal sciatic nerve. RESULTS: Distribution of microvessels and Schwann cells decreased in the DPN group (p < 0.05). NGF, TrkA and GAP-43 also decreased significantly in the DPN group (p < 0.05). NGF, TrkA, GAP-43 and distribution of microvessels and Schwann cells increased in the decompressed group (p < 0.05). DISCUSSION: In DPN rats, after nerves are compressed, microcirculation disturbance and hypoxia ischemia will happen, which cause decreased expression of NGF, TrkA and GAP-43. Finally, the self-healing function of compressed nerves is impacted. Conversely, nerve decompression can improve neural microcirculation and regeneration and change the former pathological process.
Role of acupuncture in the management of diabetic painful neuropathy (DPN): a pilot RCT.[Pubmed:24657491]
Acupunct Med. 2014 Jun;32(3):242-9.
AIMS: To examine the role of acupuncture in the treatment of diabetic painful neuropathy (DPN) using a single-blind, placebo-controlled RCT and to collect data that would be required in a future definitive study of the efficacy of acupuncture in DPN. METHODS: 45 patients were allocated to receive a 10-week course either of real (53%) or sham (47%) acupuncture. Five standardised acupuncture points on the lower limb of each leg were used in the study: LR3, KI3, SP6, SP10 and ST36. Outcome measures included the Leeds Assessment of Neuropathic Symptoms and Signs (LANSS) scale, lower limb pain (Visual Analogue Scale, VAS); Sleep Problem Scale (SPS); Measure Yourself Medical Outcome Profile (MYMOP); 36-item Short Form 36 Health Survey and resting blood pressure (BP). RESULTS: Over the 10-week treatment period, small improvements were seen in VAS -15 (-26 to -3.5), MYMOP -0.89 (-1.4 to -0.3), SPS -2.5 (-4.2 to -0.82) and resting diastolic BP -5.2 (-10.4 to -0.14) in the true acupuncture group. In contrast, there was little change in those receiving sham acupuncture. A moderate treatment effect in favour of active acupuncture was detected in MYMOP scores -0.66 (-0.96 to -0.35) but non-significant effect sizes in LANSS Pain Scale -0.37 (-2.2 to 1.4), resting diastolic BP -0.50 (-3.0 to 1.99) and the SPS -0.51 (-2.2 to 1.16). CONCLUSIONS: We have demonstrated the practicality and feasibility of acupuncture as an additional treatment for people with DPN. The treatment was well tolerated with no appreciable side effects. Larger randomised trials are needed to confirm the clinical and cost-effectiveness of acupuncture in the treatment of DPN. TRIAL REGISTRATION NUMBER: ISRCTN number: 39740785.
Effects of a combined Diesel particle filter-DeNOx system (DPN) on reactive nitrogen compounds emissions: a parameter study.[Pubmed:23214996]
Environ Sci Technol. 2012 Dec 18;46(24):13317-25.
The impact of a combined diesel particle filter-deNO(x) system (DPN) on emissions of reactive nitrogen compounds (RNCs) was studied varying the urea feed factor (alpha), temperature, and residence time, which are key parameters of the deNO(x) process. The DPN consisted of a platinum-coated cordierite filter and a vanadia-based deNO(x) catalyst supporting selective catalytic reduction (SCR) chemistry. Ammonia (NH(3)) is produced in situ from thermolysis of urea and hydrolysis of isocyanic acid (HNCO). HNCO and NH(3) are both toxic and highly reactive intermediates. The deNO(x) system was only part-time active in the ISO8178/4 C1cycle. Urea injection was stopped and restarted twice. Mean NO and NO(2) conversion efficiencies were 80%, 95%, 97% and 43%, 87%, 99%, respectively, for alpha = 0.8, 1.0, and 1.2. HNCO emissions increased from 0.028 g/h engine-out to 0.18, 0.25, and 0.26 g/h at alpha = 0.8, 1.0, and 1.2, whereas NH(3) emissions increased from <0.045 to 0.12, 1.82, and 12.8 g/h with maxima at highest temperatures and shortest residence times. Most HNCO is released at intermediate residence times (0.2-0.3 s) and temperatures (300-400 degrees C). Total RNC efficiencies are highest at alpha = 1.0, when comparable amounts of reduced and oxidized compounds are released. The DPN represents the most advanced system studied so far under the VERT protocol achieving high conversion efficiencies for particles, NO, NO(2), CO, and hydrocarbons. However, we observed a trade-off between deNO(x) efficiency and secondary emissions. Therefore, it is important to adopt such DPN technology to specific application conditions to take advantage of reduced NO(x) and particle emissions while avoiding NH(3) and HNCO slip.
Estrogen receptor alpha and beta specific agonists regulate expression of synaptic proteins in rat hippocampus.[Pubmed:19596275]
Brain Res. 2009 Sep 22;1290:1-11.
Changes in hippocampal CA1 dendritic spine density and synaptic number across the estrous cycle in female rats correlate with increased hippocampal-dependent cognitive performance in a manner that is dependent on estrogen receptors (ERs). Two isoforms of the estrogen receptor, alpha and beta are present in the rat hippocampus and distinct effects on cognitive behavior have been described for each receptor. The present study generated a profile of synaptic proteins altered by administration of estradiol benzoate, the ERalpha selective agonist PPT (1,3,5-tris (4-hydroxyphenyl)-4-propyl-1H-pyrazole) and the ERbeta selective agonist DPN (2,3-bis (4-hydroxyphenyl) propionitrile) alone and in combination in comparison to vehicle in the CA1 region of the dorsal hippocampus. In the stratum radiatum, estradiol, DPN, and PPT increased PSD-95 and AMPA-type glutamate receptor subunit GluR1. Only DPN administration regulated expression of AMPA receptor subunits GluR2 and GluR3, increasing and decreasing levels respectively. DPN also increased GluR2 expression in the other lamina of the CA1. These results support previous reports that estradiol and isoform specific agonists differentially activate ERalpha and ERbeta to regulate protein expression. The distinct effects of DPN and PPT administration on synaptic proteins suggest that the desired therapeutic outcome of estrogen may be accomplished by using specific estrogen receptor agonists. Moreover, the effects of estradiol treatment on PSD-95 expression are consistent with a growing body of evidence that this postsynaptic protein is a key marker of estrogen action related to spine synapse formation.
Activities of estrogen receptor alpha- and beta-selective ligands at diverse estrogen responsive gene sites mediating transactivation or transrepression.[Pubmed:12943986]
Mol Cell Endocrinol. 2003 Aug 29;206(1-2):13-22.
Estrogens exert their regulatory transcriptional effects, which can be stimulatory or repressive, at diverse gene sites via two estrogen receptors, ERalpha and ERbeta. Since these two ERs have different tissue distributions, ligands that have the capacity to selectively activate or inhibit these two ERs would be useful in elucidating the biology of these two receptors and might assist in the development of estrogen pharmaceuticals with improved tissue selectivity. We have developed several ligands that showed ERalpha or ERbeta selectivity at promoter-gene sites containing consensus estrogen response elements (EREs): ERalpha-selective agonist (propyl-pyrazole-triol (PPT)), ERalpha-selective antagonist (methyl-piperidino-pyrazole (MPP)), ERbeta-potency selective agonist (diarylpropionitrile (DPN)) and ERbeta-selective antagonist/ERalpha-agonist (R,R-tetrahydrochrysene (R,R-THC)). In this study, we have examined the activity of these compounds at a range of gene sites where ER stimulates gene expression through non-consensus EREs (complement C3), or multiple half-EREs (NHE-RF/EBP50), or by tethering to DNA via other proteins (TGF beta3 and progesterone receptor A/AP-1), and at gene sites where ER represses gene transcription (interleukin-6). At all of these genes, PPT showed full stimulation through ERalpha while displaying no agonism through ERbeta. MPP antagonized estradiol actions on gene transactivation and transrepression through ERalpha, with little or no effect on transcription mediated through ERbeta. DPN displayed subtype-selective agonism, being ca. 30-fold more potent through ERbeta. R,R-THC was a complete antagonist through ERbeta and displayed agonism through ERalpha, the level of which was promoter dependent. Because these ligands maintain their agonist or antagonist character and ER subtype-selectivity at gene sites of diverse nature, where estradiol is either stimulatory or inhibitory, these compounds should prove useful in elucidating the biological functions of ERalpha and ERbeta.
Acute effects of oestrogen receptor subtype-specific agonists on vascular contractility.[Pubmed:12890703]
Br J Pharmacol. 2003 Aug;139(7):1249-53.
This study shows for the first time that both the putatively selective oestrogen receptor alpha and oestrogen receptor beta agonists PPT (4,4',4"-(4-propyl-[(1)H]-pyrazole-1,3,5-triyl) tris-phenol) and DPN (2,3-bis(4-hydroxyphenyl)-propionitrile) can acutely relax precontracted isolated rat mesenteric arteries at pharmacological (i.e. micro M) concentrations. When compared to responses observed to similar concentrations of 17beta-oestrogen obtained on the same tissues, PPT had a significantly greater vasodilatory effect, while DPN had a significantly smaller effect. All responses were rapid being complete within 5 min exposure time. Thus, both PPT and DPN can acutely relax isolated mesenteric arteries with the relative potency of PPT>17beta-oestrogen>DPN.
Estrogen receptor-beta potency-selective ligands: structure-activity relationship studies of diarylpropionitriles and their acetylene and polar analogues.[Pubmed:11708925]
J Med Chem. 2001 Nov 22;44(24):4230-51.
Through an effort to develop novel ligands that have subtype selectivity for the estrogen receptors alpha (ERalpha) and beta (ERbeta), we have found that 2,3-bis(4-hydroxyphenyl)propionitrile (DPN) acts as an agonist on both ER subtypes, but has a 70-fold higher relative binding affinity and 170-fold higher relative potency in transcription assays with ERbeta than with ERalpha. To investigate the ERbeta affinity- and potency-selective character of this DPN further, we prepared a series of DPN analogues in which both the ligand core and the aromatic rings were modified by the repositioning of phenolic hydroxy groups and by the addition of alkyl substituents and nitrile groups. We also prepared other series of DPN analogues in which the nitrile functionality was replaced with acetylene groups or polar functions, to mimic the linear geometry or polarity of the nitrile, respectively. To varying degrees, all of the analogues show preferential binding affinity for ERbeta (i.e., they are ERbeta affinity-selective), and many, but not all of them, are also more potent in activating transcription through ERbeta than through ERalpha (i.e., they are ERbeta potency-selective). meso-2,3-Bis(4-hydroxyphenyl)succinonitrile and dl-2,3-bis(4-hydroxyphenyl)succinonitrile are among the highest ERbeta affinity-selective ligands, and they have an ERbeta potency selectivity that is equivalent to that of DPN. The acetylene analogues have higher binding affinities but somewhat lower selectivities than their nitrile counterparts. The polar analogues have lower affinities, and only the fluorinated polar analogues have substantial affinity selectivities. This study suggests that, in this series of ligands, the nitrile functionality is critical to ERbeta selectivity because it provides the optimal combination of linear geometry and polarity. Furthermore, the addition of a second nitrile group beta to the nitrile in DPN or the addition of a methyl substitutent at an ortho position on the beta-aromatic ring increases the affinity and selectivity of these compounds for ERbeta. These ERbeta-selective compounds may prove to be valuable tools in understanding the differences in structure and biological function of ERalpha and ERbeta.


