Roxindole hydrochlorideDopamine D2 autoreceptor agonist. Also has affinity for D3, D4, 5-HT1A receptors and the 5-HT transporter CAS# 108050-82-4 |
- Perindopril Erbumine
Catalog No.:BCC3586
CAS No.:107133-36-8
- Losartan Potassium (DuP 753)
Catalog No.:BCC1080
CAS No.:124750-99-8
- Candesartan
Catalog No.:BCC2558
CAS No.:139481-59-7
- Telmisattan
Catalog No.:BCC3863
CAS No.:144701-48-4
- Imidapril HCl
Catalog No.:BCC3792
CAS No.:89396-94-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 108050-82-4 | SDF | Download SDF |
| PubChem ID | 9886286 | Appearance | Powder |
| Formula | C23H27ClN2O | M.Wt | 382.93 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Synonyms | EMD 49980 | ||
| Solubility | Soluble to 50 mM in DMSO with gentle warming | ||
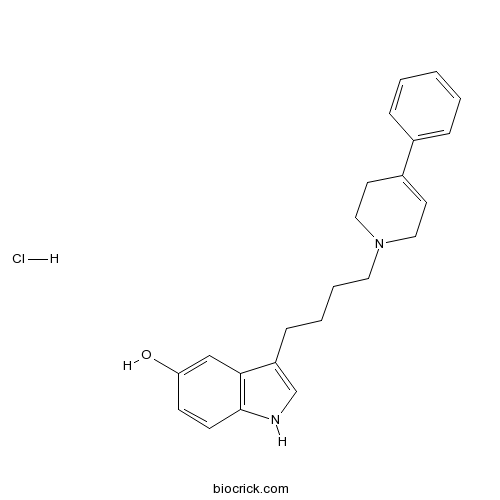
| Chemical Name | 3-[4-(4-phenyl-3,6-dihydro-2H-pyridin-1-yl)butyl]-1H-indol-5-ol;hydrochloride | ||
| SMILES | C1CN(CC=C1C2=CC=CC=C2)CCCCC3=CNC4=C3C=C(C=C4)O.Cl | ||
| Standard InChIKey | ZCEPVNSWLLJECX-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C23H26N2O.ClH/c26-21-9-10-23-22(16-21)20(17-24-23)8-4-5-13-25-14-11-19(12-15-25)18-6-2-1-3-7-18;/h1-3,6-7,9-11,16-17,24,26H,4-5,8,12-15H2;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Dopamine D2 autoreceptor agonist, with affinity for D3, D4 and 5-HT1 receptors (pKi values are 8.55, 8.93, 8.23, 9.42, 6.00 and 7.05 for human D2, D3, D4, 5-HT1A, 5-HT1B and 5-HT1D receptors). Inhibits 5-HT uptake and is antidepressant in vivo. |

Roxindole hydrochloride Dilution Calculator

Roxindole hydrochloride Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.6114 mL | 13.0572 mL | 26.1144 mL | 52.2289 mL | 65.2861 mL |
| 5 mM | 0.5223 mL | 2.6114 mL | 5.2229 mL | 10.4458 mL | 13.0572 mL |
| 10 mM | 0.2611 mL | 1.3057 mL | 2.6114 mL | 5.2229 mL | 6.5286 mL |
| 50 mM | 0.0522 mL | 0.2611 mL | 0.5223 mL | 1.0446 mL | 1.3057 mL |
| 100 mM | 0.0261 mL | 0.1306 mL | 0.2611 mL | 0.5223 mL | 0.6529 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- Tilmicosin
Catalog No.:BCC4865
CAS No.:108050-54-0
- Ambocin
Catalog No.:BCN7748
CAS No.:108044-05-9
- Bergenin monohydrate
Catalog No.:BCC8132
CAS No.:108032-11-7
- (-)-Noe's Reagent
Catalog No.:BCC8375
CAS No.:108031-79-4
- Withanolide C
Catalog No.:BCN6729
CAS No.:108030-78-0
- H-Tyr-Ome
Catalog No.:BCC3126
CAS No.:1080-06-4
- Phenol
Catalog No.:BCN3800
CAS No.:108-95-2
- Melamine
Catalog No.:BCN7248
CAS No.:108-78-1
- 6-Methyl-5,6-dihydropyran-2-one
Catalog No.:BCN3498
CAS No.:108-54-3
- Resorcinol
Catalog No.:BCN5881
CAS No.:108-46-3
- 2-(Dimethylamino)ethanol
Catalog No.:BCN1798
CAS No.:108-01-0
- Myricananin A
Catalog No.:BCN5880
CAS No.:1079941-35-7
- CP-466722
Catalog No.:BCC3912
CAS No.:1080622-86-1
- KT 5720
Catalog No.:BCC8080
CAS No.:108068-98-0
- α-Terthiophene
Catalog No.:BCN8380
CAS No.:1081-34-1
- 6-(beta-D-glucopyranosyloxy)-Salicylic acid methyl ester
Catalog No.:BCN1631
CAS No.:108124-75-0
- SKF 83566 hydrobromide
Catalog No.:BCC7121
CAS No.:108179-91-5
- TUG 424
Catalog No.:BCC7776
CAS No.:1082058-99-8
- TC-S 7005
Catalog No.:BCC6189
CAS No.:1082739-92-1
- PDE-9 inhibitor
Catalog No.:BCC1842
CAS No.:1082743-70-1
- PF-04447943
Catalog No.:BCC1850
CAS No.:1082744-20-4
- LY2584702
Catalog No.:BCC6369
CAS No.:1082949-67-4
- A 987306
Catalog No.:BCC7732
CAS No.:1082954-71-9
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
Actions of roxindole at recombinant human dopamine D2, D3 and D4 and serotonin 5-HT1A, 5-HT1B and 5-HT1D receptors.[Pubmed:10431754]
Naunyn Schmiedebergs Arch Pharmacol. 1999 Jun;359(6):447-53.
Roxindole is a potential antidepressant agent. The present study determined its affinity and agonist efficacy at recombinant human (h) dopamine hD2, hD3 and hD4 and serotonin (5-HT) h5-HT1A, h5-HT1B and h5-HT1D receptors. Roxindole exhibited high affinity at hD3 as well as at hD2 (short isoform) and hD4 (4-repeat isoform) receptors (pKi values 8.93, 8.55 and 8.23, respectively). Further, it displayed high affinity at hS-HT1A receptors (pKi = 9.42) but modest affinity at 5-HT1B and 5-HT1D receptors (pKi values 6.00 and 7.05, respectively). In [35S]GTPgammaS binding experiments, roxindole was >20-fold more potent in stimulating [35S]GTPgammaS binding at hD3 than at hD2 or hD4 receptors (pEC50 = 9.23 vs. 7.88 and 7.69). However, whereas roxindole exhibited partial agonist activity at hD3 and hD4 sites (Emax = 30.0% and 35.1%, respectively, relative to dopamine = 100%), it only weakly activated hD2 receptors (Emax = 10.5%). Roxindole potently blocked dopamine-stimulated [35S]GTPgammaS binding at hD2 receptors (pkappaB = 9.05). In comparison, the dopamine receptor agonist, (-)quinpirole, acted as a partial agonist at hD3 and hD4 sites (Emax = 67.4% and 66.3%, respectively) but surpassed the efficacy of dopamine at hD2 receptors (Emax = 132%). At h5-HT1A receptors, roxindole behaved as a high affinity (pKi = 9.42) partial agonist (Emax = 59.6%, relative to 5-HT = 100%), whereas (-)quinpirole had negligible activity. The selective 5-HT1A antagonist, WAY 100,635, blocked roxindole (100 nM)-stimulated [35S]GTPgammaS binding at h5-HT1A receptors in a concentration-dependent manner (pkappaB = 9.28). Roxindole only weakly stimulated [35S]GTPgammaS binding at 5-HT1B and 5-HT1D receptors (Emax = 27.1% and 13.7%). The present data suggest that roxindole activates mainly D3 vs. D2 or D4 receptors and 5-HT1A vs. 5-HT1B or 5-HT1D receptors. Activation of D3 and/or 5-HT1A receptors may thus contribute to its potential antidepressant properties.
Roxindole: psychopharmacological profile of a dopamine D2 autoreceptor agonist.[Pubmed:8558454]
J Pharmacol Exp Ther. 1996 Jan;276(1):41-8.
The putative, selective dopamine (DA) dopamine-2 autoreceptor agonist roxindole, which also exhibits serotonin-1A-agonistic and 5-hydroxytryptamine reuptake-inhibiting properties, was examined for its behavioral effects in rats and mice. Roxindole inhibited apomorphine-induced climbing in mice and stereotyped behavior in rats with ED50 values of 1.4 mg/kg s.c. and 0.65 mg/kg s.c., respectively, and inhibited conditioned avoidance response in rats (ED50 = 1.5 mg/kg s.c.). Thus roxindole showed a profile resembling those of the classical antipsychotic haloperidol and the atypical neuroleptic clozapine but differing from that of the DA autoreceptor agonist talipexole, which did not prevent apomorphine-induced behaviors. Unlike haloperidol, roxindole did not induce catalepsy in rats and mice. Investigations directed to the DA autoreceptor properties revealed that spontaneous motility of rats with normosensitive postsynaptic DA receptors was monophasically decreased by roxindole and talipexole, with a threshold dose of 0.0625 mg/kg s.c. for both compounds. In reserpinized rats with presumably hypersensitive postsynaptic DA receptors, roxindole only partially reversed reserpine-induced hypomotility (threshold dose: 0.25 mg/kg); talipexole re-established the activity level to that of normal rats. In contrast to apomorphine, roxindole did not induce and talipexole only marginally induced stereotyped behavior in normal rats. After administration of the DA dopamine-1 agonist SKF 38393, talipexole induced stereotyped behavior in rats, which indicated its activity at postsynaptic dopamine-2 receptors. In contrast, roxindole did not induce stereotyped behavior in rats when co-administered with SKF 38393. These results indicate that, compared with talipexole, roxindole possesses a greater selectivity for DA autoreceptors.
Biochemical and functional studies on EMD 49,980: a potent, selectively presynaptic D-2 dopamine agonist with actions on serotonin systems.[Pubmed:2565817]
Eur J Pharmacol. 1989 Jan 24;160(1):31-41.
EMD 49,980 proved to be a potent and selectively presynaptic D-2 dopamine receptor agonist in biochemical studies with rats. Thus, the gamma-butyrolactone-induced accumulation of dihydroxyphenylalanine, used as a presynaptic model, was antagonized with ED50 values of 0.29 and 0.09 mumol/kg in striatum and t. olfactorium, respectively, with high maximal effects. In contrast, striatal acetylcholine concentrations, reflecting actions at normosensitive postsynaptic D-2 receptors, were only moderately increased by about 30% over a dose range of 2.3-68 mumol/kg. In rats with unilateral nigrostriatal lesions, EMD 49,980 induced long-lasting contralateral turning, indicative of agonistic actions at denervated postsynaptic D-2 receptors. In addition, EMD 49,980 potently inhibited serotonin (5-HT) uptake in vitro and in vivo. Binding studies confirmed D-2 activity in the nM range but, similarly potent effects were observed at 5-HT1A binding sites. Measurement of 5-hydroxytryptophan (5-HTP) accumulation in the n. raphe revealed that, in vivo, the net effect of EMD 49,980 on 5-HT systems is an agonistic one. Control experiments indicate that inhibition of 5-HTP accumulation by EMD 49,980 is induced mainly via direct activation of 5-HT1A receptors, although some contribution due to 5-HT uptake inhibition is likely. Furthermore, results with various reference compounds make it unlikely that there are indirect effects, also via alpha 2-receptors in the models used and support the view that D-2 agonistic, 5-HT uptake inhibiting and 5-HT1A agonistic actions are independent properties of EMD 49,980.


