A 987306H4 receptor antagonist,potent and selective CAS# 1082954-71-9 |
- SB 431542
Catalog No.:BCC3658
CAS No.:301836-41-9
- SB-505124 hydrochloride
Catalog No.:BCC1930
CAS No.:356559-13-2
- SB525334
Catalog No.:BCC2531
CAS No.:356559-20-1
- SD-208
Catalog No.:BCC1938
CAS No.:627536-09-8
- LY2109761
Catalog No.:BCC3806
CAS No.:700874-71-1
- LY2157299
Catalog No.:BCC3709
CAS No.:700874-72-2
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1082954-71-9 | SDF | Download SDF |
| PubChem ID | 44538833 | Appearance | Powder |
| Formula | C18H25N5O | M.Wt | 327.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 100 mM in DMSO and to 100 mM in ethanol | ||
| Chemical Name | (7aR,11aR)-4-piperazin-1-yl-5,6,7a,8,9,10,11,11a-octahydro-[1]benzofuro[2,3-h]quinazolin-2-amine | ||
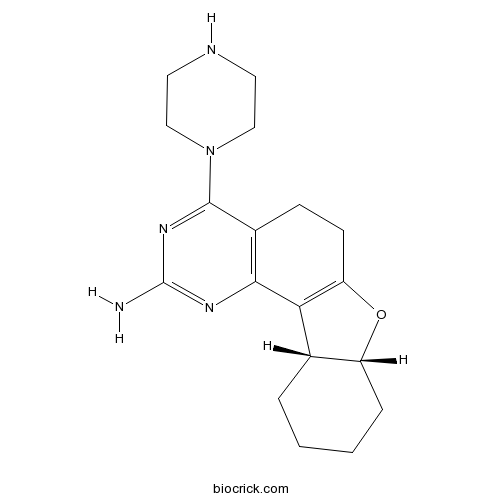
| SMILES | C1CCC2C(C1)C3=C(O2)CCC4=C3N=C(N=C4N5CCNCC5)N | ||
| Standard InChIKey | DJKJVWJQAVGLHJ-WCQYABFASA-N | ||
| Standard InChI | InChI=1S/C18H25N5O/c19-18-21-16-12(17(22-18)23-9-7-20-8-10-23)5-6-14-15(16)11-3-1-2-4-13(11)24-14/h11,13,20H,1-10H2,(H2,19,21,22)/t11-,13+/m0/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent histamine H4 receptor antagonist (pKi values are 8.24 and 8.47 in human and rat H4 receptors respectively). Displays 162-fold, 620-fold, and > 1600-fold selectivity over human H3, H1 and H2 receptors. Blocks zymosan-induced neutrophil reflux and attenuates thermal hypersensitivity in vivo (ED50 = 42 μmol/kg, ip). |

A 987306 Dilution Calculator

A 987306 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 3.0542 mL | 15.2709 mL | 30.5418 mL | 61.0836 mL | 76.3545 mL |
| 5 mM | 0.6108 mL | 3.0542 mL | 6.1084 mL | 12.2167 mL | 15.2709 mL |
| 10 mM | 0.3054 mL | 1.5271 mL | 3.0542 mL | 6.1084 mL | 7.6355 mL |
| 50 mM | 0.0611 mL | 0.3054 mL | 0.6108 mL | 1.2217 mL | 1.5271 mL |
| 100 mM | 0.0305 mL | 0.1527 mL | 0.3054 mL | 0.6108 mL | 0.7635 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
A987306,cis-4-(Piperazin-1-yl)-5,6,7a,8,9,10,11,11a-octahydrobenzofuro[2,3-h]quin- azolin-2-amine[1], is a selective and potent H4 receptor antagonist [2].
H4R is one of 4 known G-protein-coupled receptors (H1, H2, H3 and H4 receptors) of histamine. It is for mediating some physiological functions of histamine [3].
In a cell-based Ca2+-flux functional assay, A 987306 had no activation to the receptor, but blocked the H4R activation induced by endogenous histamine. A 987306 potently decreased histamine-mediated binding between rat H4-receptor-containing membranes and GTP-γ-[35S] with a Kb of 6 nM [3].
In Sprague-Dawley rats, after ip injection, A 987306 had a favorable fractional bioavailability (Fip/iv =72%), a half-life of 4.7 h and a Cmax of 1.73 μM at a Tmax of 0.25 h after dosing. After oral dosing, A 987306 had a moderate fractional oral bioavailability (Fpo/iv =26%) with a half-life of 3.7 h and a Cmax of 0.30 μM at a Tmax of 1.5 h after dosing. The plasma protein binding of A 987306 measured in rats was found to be 59% [3].
To human H4R and rat H4R, the Ki values of A 987306 are 5.8 nM and 3.4 nM, respectively. A 987306 reduced scratch responses in mice with an ED50 of 0.36 μmol/kg. A 987306 was found to be selective and bear an IC50 > 810 nM for over 100 kinases [3].
References:
[1]. Vanina A. Medina and Elena S. Rivera. Histamine receptors and cancer pharmacology. British Journal of Pharmacology, 2010, 161:755-767.
[2]. M.I. Strakhova, C.A. Cuff, A.M. Manelli, et al. In vitro and in vivo characterization of A-940894: a potent histamine H4 receptor antagonist with anti-inflammatory properties. British Journal of Pharmacology, 2009, 157:44-54.
[3]. Huaqing Liu, Robert J. Altenbach, Tracy L. Carr, et al. cis-4-(Piperazin-1-yl)-5,6,7a,8,9,10,11,11a-octahydrobenzofuro[2,3-h]quinazolin-2-amine (A-987306), A New Histamine H4R Antagonist that Blocks Pain Responses against Carrageenan-Induced Hyperalgesia. J. Med. Chem., 2008, 51:7094-7098.
- LY2584702
Catalog No.:BCC6369
CAS No.:1082949-67-4
- PF-04447943
Catalog No.:BCC1850
CAS No.:1082744-20-4
- PDE-9 inhibitor
Catalog No.:BCC1842
CAS No.:1082743-70-1
- TC-S 7005
Catalog No.:BCC6189
CAS No.:1082739-92-1
- TUG 424
Catalog No.:BCC7776
CAS No.:1082058-99-8
- SKF 83566 hydrobromide
Catalog No.:BCC7121
CAS No.:108179-91-5
- 6-(beta-D-glucopyranosyloxy)-Salicylic acid methyl ester
Catalog No.:BCN1631
CAS No.:108124-75-0
- α-Terthiophene
Catalog No.:BCN8380
CAS No.:1081-34-1
- KT 5720
Catalog No.:BCC8080
CAS No.:108068-98-0
- CP-466722
Catalog No.:BCC3912
CAS No.:1080622-86-1
- Roxindole hydrochloride
Catalog No.:BCC7116
CAS No.:108050-82-4
- Tilmicosin
Catalog No.:BCC4865
CAS No.:108050-54-0
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
- 1,7-Bis(4-hydroxyphenyl)hept-6-en-3-ol
Catalog No.:BCN1630
CAS No.:1083195-05-4
- 1,7-Bis(4-hydroxyphenyl)hept-1-en-3-one
Catalog No.:BCN1629
CAS No.:1083200-79-6
- Fmoc-D-Asn-OH
Catalog No.:BCC3083
CAS No.:108321-39-7
- Geneticin, G-418 Sulfate
Catalog No.:BCC1202
CAS No.:108321-42-2
- Ganoderic acid D
Catalog No.:BCN2437
CAS No.:108340-60-9
- Noradrenaline bitartrate monohydrate
Catalog No.:BCC4810
CAS No.:108341-18-0
- Fuligorubin A
Catalog No.:BCN1837
CAS No.:108343-55-1
- FH535
Catalog No.:BCC1573
CAS No.:108409-83-2
- [D-Phe12]-Bombesin
Catalog No.:BCC5844
CAS No.:108437-87-2
- [D-Phe12,Leu14]-Bombesin
Catalog No.:BCC6020
CAS No.:108437-88-3
- Ilexsaponin A
Catalog No.:BCN7867
CAS No.:108524-93-2
cis-4-(Piperazin-1-yl)-5,6,7a,8,9,10,11,11a-octahydrobenzofuro[2,3-h]quinazolin-2 -amine (A-987306), a new histamine H4R antagonist that blocks pain responses against carrageenan-induced hyperalgesia.[Pubmed:18983139]
J Med Chem. 2008 Nov 27;51(22):7094-8.
cis-4-(Piperazin-1-yl)-5,6,7a,8,9,10,11,11a-octahydrobenzofuro[2,3-h]quinazolin-2 -amine, 4 (A-987306) is a new histamine H(4) antagonist. The compound is potent in H(4) receptor binding assays (rat H(4), K(i) = 3.4 nM, human H(4) K(i) = 5.8 nM) and demonstrated potent functional antagonism in vitro at human, rat, and mouse H(4) receptors in cell-based FLIPR assays. Compound 4 also demonstrated H(4) antagonism in vivo in mice, blocking H(4)-agonist induced scratch responses, and showed anti-inflammatory activity in mice in a peritonitis model. Most interesting was the high potency and efficacy of this compound in blocking pain responses, where it showed an ED(50) of 42 mumol/kg (ip) in a rat post-carrageenan thermal hyperalgesia model of inflammatory pain.
Molecular and biochemical pharmacology of the histamine H4 receptor.[Pubmed:19413568]
Br J Pharmacol. 2009 May;157(1):14-23.
The elucidation of the human genome has had a major impact on histamine receptor research. The identification of the human H4 receptor by several groups has been instrumental for a new appreciation of the role of histamine in the modulation of immune function. In this review, we summarize the historical developments and the molecular and biochemical pharmacology of the H4 receptor.


