SKF 83566 hydrobromideSelectively inhibits AC2; also a potent, selective D1-like antagonist CAS# 108179-91-5 |
- SB202190 (FHPI)
Catalog No.:BCC1093
CAS No.:152121-30-7
- SB 239063
Catalog No.:BCC1923
CAS No.:193551-21-2
- SD-06
Catalog No.:BCC1937
CAS No.:271576-80-8
- BIRB 796 (Doramapimod)
Catalog No.:BCC2535
CAS No.:285983-48-4
- LY2228820
Catalog No.:BCC2528
CAS No.:862507-23-1
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 108179-91-5 | SDF | Download SDF |
| PubChem ID | 23581817 | Appearance | Powder |
| Formula | C17H19Br2NO | M.Wt | 413.15 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 5 mM in water with gentle warming and to 100 mM in DMSO | ||
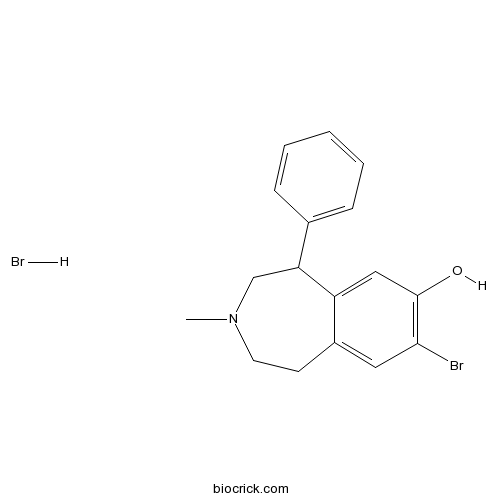
| Chemical Name | 8-bromo-3-methyl-5-phenyl-1,2,4,5-tetrahydro-3-benzazepin-7-ol;hydrobromide | ||
| SMILES | CN1CCC2=CC(=C(C=C2C(C1)C3=CC=CC=C3)O)Br.Br | ||
| Standard InChIKey | SDQJYYGODYRPBR-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C17H18BrNO.BrH/c1-19-8-7-13-9-16(18)17(20)10-14(13)15(11-19)12-5-3-2-4-6-12;/h2-6,9-10,15,20H,7-8,11H2,1H3;1H | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Potent and selective D1-like dopamine receptor antagonist (Ki~ 0.56 nM for D1; KB = 2 μM for D2). Also antagonist at the vascular 5-HT2 receptor (Ki = 11 nM). Displays selective inhibition of adenylyl cyclase 2 (AC2); inactive against AC1 or AC5. Centrally active following systemic administration in vivo. |

SKF 83566 hydrobromide Dilution Calculator

SKF 83566 hydrobromide Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.4204 mL | 12.1021 mL | 24.2043 mL | 48.4086 mL | 60.5107 mL |
| 5 mM | 0.4841 mL | 2.4204 mL | 4.8409 mL | 9.6817 mL | 12.1021 mL |
| 10 mM | 0.242 mL | 1.2102 mL | 2.4204 mL | 4.8409 mL | 6.0511 mL |
| 50 mM | 0.0484 mL | 0.242 mL | 0.4841 mL | 0.9682 mL | 1.2102 mL |
| 100 mM | 0.0242 mL | 0.121 mL | 0.242 mL | 0.4841 mL | 0.6051 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
- 6-(beta-D-glucopyranosyloxy)-Salicylic acid methyl ester
Catalog No.:BCN1631
CAS No.:108124-75-0
- α-Terthiophene
Catalog No.:BCN8380
CAS No.:1081-34-1
- KT 5720
Catalog No.:BCC8080
CAS No.:108068-98-0
- CP-466722
Catalog No.:BCC3912
CAS No.:1080622-86-1
- Roxindole hydrochloride
Catalog No.:BCC7116
CAS No.:108050-82-4
- Tilmicosin
Catalog No.:BCC4865
CAS No.:108050-54-0
- Ambocin
Catalog No.:BCN7748
CAS No.:108044-05-9
- Bergenin monohydrate
Catalog No.:BCC8132
CAS No.:108032-11-7
- (-)-Noe's Reagent
Catalog No.:BCC8375
CAS No.:108031-79-4
- Withanolide C
Catalog No.:BCN6729
CAS No.:108030-78-0
- H-Tyr-Ome
Catalog No.:BCC3126
CAS No.:1080-06-4
- Phenol
Catalog No.:BCN3800
CAS No.:108-95-2
- TUG 424
Catalog No.:BCC7776
CAS No.:1082058-99-8
- TC-S 7005
Catalog No.:BCC6189
CAS No.:1082739-92-1
- PDE-9 inhibitor
Catalog No.:BCC1842
CAS No.:1082743-70-1
- PF-04447943
Catalog No.:BCC1850
CAS No.:1082744-20-4
- LY2584702
Catalog No.:BCC6369
CAS No.:1082949-67-4
- A 987306
Catalog No.:BCC7732
CAS No.:1082954-71-9
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
- 1,7-Bis(4-hydroxyphenyl)hept-6-en-3-ol
Catalog No.:BCN1630
CAS No.:1083195-05-4
- 1,7-Bis(4-hydroxyphenyl)hept-1-en-3-one
Catalog No.:BCN1629
CAS No.:1083200-79-6
- Fmoc-D-Asn-OH
Catalog No.:BCC3083
CAS No.:108321-39-7
- Geneticin, G-418 Sulfate
Catalog No.:BCC1202
CAS No.:108321-42-2
- Ganoderic acid D
Catalog No.:BCN2437
CAS No.:108340-60-9
D1 receptor antagonist-induced long-term depression in the medial prefrontal cortex of rat, in vivo: an animal model of psychiatric hypofrontality.[Pubmed:18635697]
J Psychopharmacol. 2009 Aug;23(6):672-85.
The objective of the following experiment was to induce a pathogenic hypofrontal condition by administering a dopamine-1 receptor (D(1)R) antagonist to rats. The pathophysiological effect of this manipulation upon glutamate-based long-term potentiation (LTP) in the medial prefrontal cortex (mPFC) was examined in vivo. Subjects were surgically implanted with stimulating electrodes into the corpus callosum and recording electrodes into the mPFC. High-frequency stimulation (HFS) was combined with the administration of the selective D(1)R family agonist A68930 hydrochloride (0.4 mg/kg/mL) and the selective D(1)R family antagonist SKF 83566 (0.15 mg/kg/mL). The administration of SKF 83566 hydrobromide prevented mPFC LTP, and resulted in HFS-induced long-term depression. This indicates that D(1)R activation is necessary for the induction of mPFC glutamate-based LTP. This is supported by our finding that the administration of A68930 hydrochloride combined with HFS induced LTP comparable with saline control levels, suggesting that D(1)R activation is necessary for the induction of baseline levels of mPFC LTP. Given that the mPFC governs executive behaviours that are subserved by LTP, such as working memory, these findings are relevant for the study of psychopathological conditions in which hypodopaminergic conditions exist in the mPFC and are correlated with psychiatric symptomotology, such as drug addiction and schizophrenia.
Development of a high-throughput screening paradigm for the discovery of small-molecule modulators of adenylyl cyclase: identification of an adenylyl cyclase 2 inhibitor.[Pubmed:24008337]
J Pharmacol Exp Ther. 2013 Nov;347(2):276-87.
Adenylyl cyclase (AC) isoforms are implicated in several physiologic processes and disease states, but advancements in the therapeutic targeting of AC isoforms have been limited by the lack of potent and isoform-selective small-molecule modulators. The discovery of AC isoform-selective small molecules is expected to facilitate the validation of AC isoforms as therapeutic targets and augment the study of AC isoform function in vivo. Identification of chemical probes for AC2 is particularly important because there are no published genetic deletion studies and few small-molecule modulators. The present report describes the development and implementation of an intact-cell, small-molecule screening approach and subsequent validation paradigm for the discovery of AC2 inhibitors. The NIH clinical collections I and II were screened for inhibitors of AC2 activity using PMA-stimulated cAMP accumulation as a functional readout. Active compounds were subsequently confirmed and validated as direct AC2 inhibitors using orthogonal and counterscreening assays. The screening effort identified SKF-83566 [8-bromo-2,3,4,5-tetrahydro-3-methyl-5-phenyl-1H-3-benzazepin-7-ol hydrobromide] as a selective AC2 inhibitor with superior pharmacological properties for selective modulation of AC2 compared with currently available AC inhibitors. The utility of SKF-83566 as a small-molecule probe to study the function of endogenous ACs was demonstrated in C2C12 mouse skeletal muscle cells and human bronchial smooth muscle cells.
Locomotor stereotypy produced by dexbenzetimide and scopolamine is reduced by SKF 83566, not sulpiride.[Pubmed:9678647]
Pharmacol Biochem Behav. 1998 Jul;60(3):639-44.
Like amphetamine, scopolamine produces locomotor stereotypy (repetitive routes of locomotion) in an open field. To determine whether locomotor stereotypy is a common behavioral effect of anticholingeric agents, several doses of the anticholinergic dexbenzetimide were tested for the ability to produce locomotor stereotypy; like scopolamine, dexbenzetimide produced locomotor stereotypy. To investigate a possible role of dopamine in anticholinergic-induced locomotor stereotypy, we tested the ability of the dopamine D1 antagonist SKF 83566 and the D2 antagonist sulpiride to block the locomotor stereotypy induced by scopolamine as well as dexbenzetimide. SKF 83566 blocked scopolamine- and dexbenzetimide-induced locomotor stereotypy; sulpiride did not reduce dexbenzetimide-induced locomotor stereotypy, but enhanced scopolamine-induced locomotor stereotypy. Hyperlocomotion was reduced by both dopamine antagonists. Results are interpreted in support of the notion that dopamine is the likely candidate mediating locomotor stereotypy.
Effects of dopamine D1 antagonists SCH23390 and SK&F83566 on locomotor activities in rats.[Pubmed:8446676]
Pharmacol Biochem Behav. 1993 Feb;44(2):429-32.
The effects of the dopamine D1 antagonists R-(+)-7-chloro-8-hydroxy-3-methyl-1phenyl-2,3,4,5-tetrahydro-1-H-3 -benzazapine (SCH23390) and (+-)-7-bromo-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1- H-3-benzazapine (SK&F83566) were tested for 2 h on linear locomotor, rearing, stereotypy, and margin times in an open field. Each of the antagonists attenuated the duration of linear locomotion, rearing, and stereotypy times in a dose- and time-dependent manner. The effectiveness of the antagonists was relatively brief and SCH23390 was more effective than SK&F83566 on each behavior. The two antagonists had differential effects on margin time.
SCH 23390 and SK&F 83566 are antagonists at vascular dopamine and serotonin receptors.[Pubmed:3884345]
Eur J Pharmacol. 1985 Jan 22;108(2):205-8.
SCH 23390 and SK&F 83566 have been utilized as selective antagonists at postjunctional dopamine receptors. However, in the isolated rabbit thoracic aorta evidence for competitive antagonism of serotonin was obtained. The KB values were 11 and 34 nM for SK&F R-83566 and SCH 23390, respectively. The S-enantiomer of SK&F 83566 was a weaker antagonist at the vascular serotonin receptor (KB = 1.5 microM). Thus, these results indicate that SCH 23390 and SK&F 83566 are not totally specific for the dopamine DA-1 receptor because they can also be potent antagonists at the vascular serotonin receptor.


