LY2584702p70 S6 kinase inhibitor CAS# 1082949-67-4 |
- CX-5461
Catalog No.:BCC3700
CAS No.:1138549-36-6
- Gemcitabine HCl
Catalog No.:BCC1076
CAS No.:122111-03-9
- Fludarabine Phosphate (Fludara)
Catalog No.:BCC3681
CAS No.:75607-67-9
- Bleomycin Sulfate
Catalog No.:BCC3694
CAS No.:9041-93-4
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 1082949-67-4 | SDF | Download SDF |
| PubChem ID | 25118925 | Appearance | Powder |
| Formula | C21H19F4N7 | M.Wt | 445.42 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | DMSO : ≥ 4.5 mg/mL (10.10 mM) *"≥" means soluble, but saturation unknown. | ||
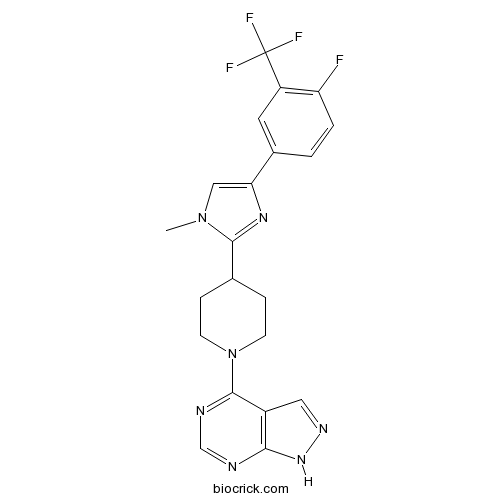
| Chemical Name | 4-[4-[4-[4-fluoro-3-(trifluoromethyl)phenyl]-1-methylimidazol-2-yl]piperidin-1-yl]-1H-pyrazolo[3,4-d]pyrimidine | ||
| SMILES | CN1C=C(N=C1C2CCN(CC2)C3=NC=NC4=C3C=NN4)C5=CC(=C(C=C5)F)C(F)(F)F | ||
| Standard InChIKey | FYXRSVDHGLUMHB-UHFFFAOYSA-N | ||
| Standard InChI | InChI=1S/C21H19F4N7/c1-31-10-17(13-2-3-16(22)15(8-13)21(23,24)25)29-19(31)12-4-6-32(7-5-12)20-14-9-28-30-18(14)26-11-27-20/h2-3,8-12H,4-7H2,1H3,(H,26,27,28,30) | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | LY-2584702 is an orally available inhibitor of p70S6K signaling; inhibits p70S6K and prevents phosphorylation of the S6 subunit of ribosomes.
IC50 value:
Target: p70S6K inhibitor
LY-2584702 is an orally available inhibitor of p70S6K signaling, with potential antineoplastic activity. LY2584702 inhibits ribosomal protein S6 Kinase (p70S6K), and prevents phosphorylation of the S6 subunit of ribosomes, thereby inhibiting normal ribosomal function within tumor cells leading to a decrease in protein synthesis and in cellular proliferation. P70S6K, a serine/threonine kinase, acts downstream of PIP3 and phosphoinositide-dependent kinase-1 in the PI3 kinase pathway, is often upregulated in a variety of cancer cells, and is involved in the regulation of cell growth, proliferation, motility, and survival. References: | |||||

LY2584702 Dilution Calculator

LY2584702 Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 2.2451 mL | 11.2254 mL | 22.4507 mL | 44.9014 mL | 56.1268 mL |
| 5 mM | 0.449 mL | 2.2451 mL | 4.4901 mL | 8.9803 mL | 11.2254 mL |
| 10 mM | 0.2245 mL | 1.1225 mL | 2.2451 mL | 4.4901 mL | 5.6127 mL |
| 50 mM | 0.0449 mL | 0.2245 mL | 0.449 mL | 0.898 mL | 1.1225 mL |
| 100 mM | 0.0225 mL | 0.1123 mL | 0.2245 mL | 0.449 mL | 0.5613 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
LY2584702 is a potent and selective inhibitor of p70 S6 kinase with IC50 value of 4 nM [1].
P70 S6 kinase (p70S6K) is a serine/threonine protein kinase that acts downstream of the phosphatidylinositol-3-kinase (PI3K)/Akt/mTOR signalling pathway. p70S6K phosphorylates the eukaryotic initiation factor 4B (eIF4B), a regulator of protein synthesis, and phosphorylates and activates ribosomal protein S6 (S6), a component of the 40S ribosomal subunit [1] [2].
LY2584702 is an oral and selective ATP competitive p70S6K inhibitor. In HCT116 colon cancer cells, LY2584702 inhibits phosphorylation of S6 (pS6) with IC50 value of 0.1-0.24 μM [1].
In the HCT116 colon carcinoma xenograft model, LY2584702 significantly inhibited tumour growth with threshold minimum effective dose 50% (TMED50) and TMED90 of 2.3 mg/kg and 10 mg/kg, respectively. In both HCT116 colon carcinoma and U87MG glioblastoma xenograft models, LY2584702 at levels of 2.5 mg/kg twice daily (BID) and 12.5 mg/kg BID exhibited significant efficacy. Treatment patients with advanced solid tumours with LY2584702 orally on a 28-day cycle, the maximum tolerated dose (MTD) was 75 mg BID (twice-daily) or 100 mg QD (once-daily) [1].
References:
[1]. Tolcher A, Goldman J, Patnaik A, et al. A phase I trial of LY2584702 tosylate, a p70 S6 kinase inhibitor, in patients with advanced solid tumours. Eur J Cancer, 2014, 50(5): 867-875.
[2]. Hollebecque A, Houédé N, Cohen EE, et al. A phase Ib trial of LY2584702 tosylate, a p70 S6 inhibitor, in combination with erlotinib or everolimus in patients with solid tumours. Eur J Cancer, 2014, 50(5): 876-884.
- PF-04447943
Catalog No.:BCC1850
CAS No.:1082744-20-4
- PDE-9 inhibitor
Catalog No.:BCC1842
CAS No.:1082743-70-1
- TC-S 7005
Catalog No.:BCC6189
CAS No.:1082739-92-1
- TUG 424
Catalog No.:BCC7776
CAS No.:1082058-99-8
- SKF 83566 hydrobromide
Catalog No.:BCC7121
CAS No.:108179-91-5
- 6-(beta-D-glucopyranosyloxy)-Salicylic acid methyl ester
Catalog No.:BCN1631
CAS No.:108124-75-0
- α-Terthiophene
Catalog No.:BCN8380
CAS No.:1081-34-1
- KT 5720
Catalog No.:BCC8080
CAS No.:108068-98-0
- CP-466722
Catalog No.:BCC3912
CAS No.:1080622-86-1
- Roxindole hydrochloride
Catalog No.:BCC7116
CAS No.:108050-82-4
- Tilmicosin
Catalog No.:BCC4865
CAS No.:108050-54-0
- Ambocin
Catalog No.:BCN7748
CAS No.:108044-05-9
- A 987306
Catalog No.:BCC7732
CAS No.:1082954-71-9
- Cariprazine hydrochloride
Catalog No.:BCC1454
CAS No.:1083076-69-0
- 1,7-Bis(4-hydroxyphenyl)hept-6-en-3-ol
Catalog No.:BCN1630
CAS No.:1083195-05-4
- 1,7-Bis(4-hydroxyphenyl)hept-1-en-3-one
Catalog No.:BCN1629
CAS No.:1083200-79-6
- Fmoc-D-Asn-OH
Catalog No.:BCC3083
CAS No.:108321-39-7
- Geneticin, G-418 Sulfate
Catalog No.:BCC1202
CAS No.:108321-42-2
- Ganoderic acid D
Catalog No.:BCN2437
CAS No.:108340-60-9
- Noradrenaline bitartrate monohydrate
Catalog No.:BCC4810
CAS No.:108341-18-0
- Fuligorubin A
Catalog No.:BCN1837
CAS No.:108343-55-1
- FH535
Catalog No.:BCC1573
CAS No.:108409-83-2
- [D-Phe12]-Bombesin
Catalog No.:BCC5844
CAS No.:108437-87-2
- [D-Phe12,Leu14]-Bombesin
Catalog No.:BCC6020
CAS No.:108437-88-3
A phase Ib trial of LY2584702 tosylate, a p70 S6 inhibitor, in combination with erlotinib or everolimus in patients with solid tumours.[Pubmed:24456794]
Eur J Cancer. 2014 Mar;50(5):876-84.
BACKGROUND: LY2584702 tosylate (hereafter referred to as LY2584702) is an oral, selective ATP competitive inhibitor of p70 S6 kinase. Preclinical studies with LY2584702 demonstrated significant synergistic activity with erlotinib and everolimus. The primary objective was to determine a phase II dose and schedule. Secondary objectives included evaluation of safety, toxicity and pharmacokinetics of LY2584702 in combination with erlotinib or everolimus. METHODS: Patients with advanced solid tumours were treated with a total daily dose of 50-200mg of LY2584702 in combination with erlotinib 150 mg once daily (Arm A) or everolimus 10mg once daily (Arm B). Dose escalation was based on 3+3 design and used the Common Terminology Criteria for Adverse Events Version 4.0. RESULTS: Twenty-nine patients were enrolled, 17 in Arm A and 12 in Arm B. Dose limiting toxicities (DLTs) in cycle 1 were observed in Arm A in four patients and consisted of Grade 3 vomiting, hypophosphataemia, pulmonary embolism and decreased clotting factor V. No DLTs were observed in Arm B at cycle 1, and the most frequent treatment-emergent adverse events related to study drug were: fatigue, anorexia, diarrhoea, nausea and vomiting. Seven patients received >/=4 cycles (3 in A, 4 in B). Best overall response was stable disease. Exposure accumulation of LY2584702 occurred with BID (twice daily) dosing. Exposure of erlotinib increased when administered in combination with LY2584702. CONCLUSION: LY2584702 was not well tolerated when administered with erlotinib, therefore this combination is not feasible. The combination with everolimus was better tolerated but yielded very limited clinical benefit.
A phase I trial of LY2584702 tosylate, a p70 S6 kinase inhibitor, in patients with advanced solid tumours.[Pubmed:24440085]
Eur J Cancer. 2014 Mar;50(5):867-75.
BACKGROUND: LY2584702 tosylate (hereafter referred to as LY2584702) is a potent, highly selective adenosine triphosphate (ATP) competitive inhibitor against p70 S6 kinase, a downstream component of the phosphatidylinositol-3-kinase signalling pathway which regulates cell proliferation and survival. LY2584702 exhibited anti-tumour activity in preclinical analysis. METHODS: Patients with advanced solid tumours were treated with LY2584702 orally on a 28-day cycle until the criteria for maximum tolerated dose (MTD) were met. Skin biopsies were collected for pharmacodynamic analysis, and levels of phospho-S6 protein were examined. The primary objective was to determine a phase II dose and schedule with secondary objectives of observing safety and tolerability. Dose escalation was based upon Common Terminology Criteria for Adverse Events Version 3.0. RESULTS: Thirty-four patients were enrolled onto this phase I study and treated with LY2584702 on a QD (once-daily) or BID (twice-daily) dosing schedule. Part A dose escalation (n=22) began with 300 mg BID (n=2). Due to toxicity, this was scaled back to doses of 25mg (n=3), 50 mg (n=8), 100mg (n=3), and 200 mg (n=6) QD. Part B dose escalation (n=12) included 50 mg (n=3), 75 mg (n=3), and 100 mg (n=6) BID. Seven patients experienced dose-limiting toxicity (DLT). All DLTs were Grade 3 and included vomiting, increased lipase, nausea, hypophosphataemia, fatigue and pancreatitis. CONCLUSION: The MTD was determined to be 75 mg BID or 100mg QD. No responses were observed at these levels. Pharmacokinetic analysis revealed substantial variability in exposure and determined that LY2584702 treatment was not dose proportional with increasing dose.
Leukotriene B(4) Metabolism and p70S6 Kinase 1 Inhibitors: PF-4708671 but Not LY2584702 Inhibits CYP4F3A and the omega-Oxidation of Leukotriene B(4) In Vitro and In Cellulo.[Pubmed:28068410]
PLoS One. 2017 Jan 9;12(1):e0169804.
LTB4 is an inflammatory lipid mediator mainly biosynthesized by leukocytes. Since its implication in inflammatory diseases is well recognized, many tools to regulate its biosynthesis have been developed and showed promising results in vitro and in vivo, but mixed results in clinical trials. Recently, the mTOR pathway component p70S6 kinase 1 (p70S6K1) has been linked to LTC4 synthase and the biosynthesis of cysteinyl-leukotrienes. In this respect, we investigated if p70S6K1 could also play a role in LTB4 biosynthesis. We thus evaluated the impact of the p70S6K1 inhibitors PF-4708671 and LY2584702 on LTB4 biosynthesis in human neutrophils. At a concentration of 10 muM, both compounds inhibited S6 phosphorylation, although neither one inhibited the thapsigargin-induced LTB4 biosynthesis, as assessed by the sum of LTB4, 20-OH-LTB4, and 20-COOH-LTB4. However, PF-4708671, but not LY2584702, inhibited the omega-oxidation of LTB4 into 20-OH-LTB4 by intact neutrophils and by recombinant CYP4F3A, leading to increased LTB4 levels. This was true for both endogenously biosynthesized and exogenously added LTB4. In contrast to that of 17-octadecynoic acid, the inhibitory effect of PF-4708671 was easily removed by washing the neutrophils, indicating that PF-4708671 was a reversible CYP4F3A inhibitor. At optimal concentration, PF-4708671 increased the half-life of LTB4 in our neutrophil suspensions by 7.5 fold, compared to 5 fold for 17-octadecynoic acid. Finally, Michaelis-Menten and Lineweaver-Burk plots indicate that PF-4708671 is a mixed inhibitor of CYP4F3A. In conclusion, we show that PF-4708671 inhibits CYP4F3A and prevents the omega-oxidation of LTB4 in cellulo, which might result in increased LTB4 levels in vivo.


