(-)-Terreic acidantibiotic activity,inhibits protein synthesis CAS# 121-40-4 |
Quality Control & MSDS
3D structure
Package In Stock
Number of papers citing our products

| Cas No. | 121-40-4 | SDF | Download SDF |
| PubChem ID | 91437 | Appearance | Powder |
| Formula | C7H6O4 | M.Wt | 154.12 |
| Type of Compound | N/A | Storage | Desiccate at -20°C |
| Solubility | Soluble to 20 mM in water | ||
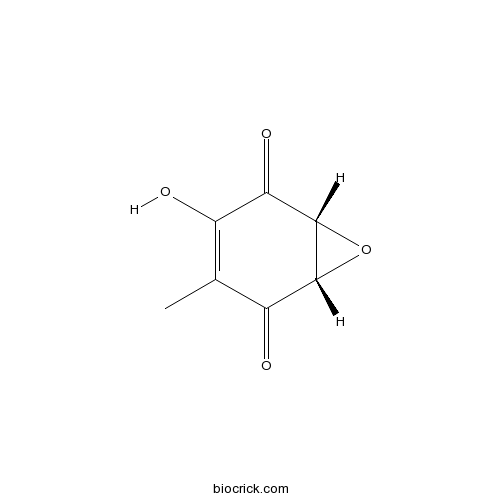
| Chemical Name | (1S,6R)-4-hydroxy-3-methyl-7-oxabicyclo[4.1.0]hept-3-ene-2,5-dione | ||
| SMILES | CC1=C(C(=O)C2C(C1=O)O2)O | ||
| Standard InChIKey | ATFNSNUJZOYXFC-RQJHMYQMSA-N | ||
| Standard InChI | InChI=1S/C7H6O4/c1-2-3(8)5(10)7-6(11-7)4(2)9/h6-8H,1H3/t6-,7+/m1/s1 | ||
| General tips | For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months. We recommend that you prepare and use the solution on the same day. However, if the test schedule requires, the stock solutions can be prepared in advance, and the stock solution must be sealed and stored below -20℃. In general, the stock solution can be kept for several months. Before use, we recommend that you leave the vial at room temperature for at least an hour before opening it. |
||
| About Packaging | 1. The packaging of the product may be reversed during transportation, cause the high purity compounds to adhere to the neck or cap of the vial.Take the vail out of its packaging and shake gently until the compounds fall to the bottom of the vial. 2. For liquid products, please centrifuge at 500xg to gather the liquid to the bottom of the vial. 3. Try to avoid loss or contamination during the experiment. |
||
| Shipping Condition | Packaging according to customer requirements(5mg, 10mg, 20mg and more). Ship via FedEx, DHL, UPS, EMS or other couriers with RT, or blue ice upon request. | ||
| Description | Selective inhibitor of Bruton's tyrosine kinase (BTK). Inhibits the interaction between PKCbII and BTK (IC50 ~ 30 mM) and the catalytic activity of BTK but does not affect the activity of PKC. Has little effect on the activities of Lyn, Syk, PKA, casein kinase I, ERK1, ERK2 and p38 kinases. |

(-)-Terreic acid Dilution Calculator

(-)-Terreic acid Molarity Calculator
| 1 mg | 5 mg | 10 mg | 20 mg | 25 mg | |
| 1 mM | 6.4885 mL | 32.4423 mL | 64.8845 mL | 129.769 mL | 162.2113 mL |
| 5 mM | 1.2977 mL | 6.4885 mL | 12.9769 mL | 25.9538 mL | 32.4423 mL |
| 10 mM | 0.6488 mL | 3.2442 mL | 6.4885 mL | 12.9769 mL | 16.2211 mL |
| 50 mM | 0.1298 mL | 0.6488 mL | 1.2977 mL | 2.5954 mL | 3.2442 mL |
| 100 mM | 0.0649 mL | 0.3244 mL | 0.6488 mL | 1.2977 mL | 1.6221 mL |
| * Note: If you are in the process of experiment, it's necessary to make the dilution ratios of the samples. The dilution data above is only for reference. Normally, it's can get a better solubility within lower of Concentrations. | |||||

Calcutta University

University of Minnesota

University of Maryland School of Medicine

University of Illinois at Chicago

The Ohio State University

University of Zurich

Harvard University

Colorado State University

Auburn University

Yale University

Worcester Polytechnic Institute

Washington State University

Stanford University

University of Leipzig

Universidade da Beira Interior

The Institute of Cancer Research

Heidelberg University

University of Amsterdam

University of Auckland

TsingHua University

The University of Michigan

Miami University

DRURY University

Jilin University

Fudan University

Wuhan University

Sun Yat-sen University

Universite de Paris

Deemed University

Auckland University

The University of Tokyo

Korea University
IC50: about 100 μM for binding of GST-BtkPH to PKC, about 30 μM for association of Btk with PKCbII, about 10 μM for JNK1 activity, and about 7 μM for TNF-a and about 3 μM for IL-2.
Terreic acid (TA), which was known as Aspergillus sp. No. Y-8980, was isolated from a soil sample abtained at Yoron Island of Kagoshima Prefecture.
Terreic acid is a quinone epoxide with antibiotic activity. At a mechanism view for its antibiotic action, it inhibits protein synthesis by blocking the formation of leucyl-tRNA in sensitive bacteria [1]. TA was also an inhibitor of Bruton’s tyrosine kinase (Btk) that blocks the PKC-Btk PH domain interaction. Btk plays core roles in mast cell activation and in B cell development [2].
In vitro: Terreic acid showed MIC (minimal inhibitory concentration) of 25 approximately 100 mcg/ml, 50 mcg/ml and 12.5 mcg/ml against Gram-positive and Gram-negative bacteria, Xanthomonas citri and Xanthomonas oryzae and, respectively. TA also showed anti-tumor effect in the concentrations of > 6.25 mcg/ml on human carcinoma cells (HeLa cells) [1]. In lysates of HMC-1 human mast cells, TA can inhibit the binding of GST-BtkPH to PKC with an IC50 of ~100 uM. In mouse mast cells, TA can inhibit the association of Btk with PKCbII at IC50 of ~ 30 uM. JNK1 activity can be inhibited by TA with an IC50 of ~10 uM. Cytokine secretion was severely impaired by TA with an IC50 of ~7 uM for TNF-a and ~ 3 uM for IL-2) [2].
In vivo: In mice, the LD50 (median lethal dose) of TA was 75 mg/kg through i.p. and i.v. TA showed the enough survival effect in dd mice which have been implanted with Ehrlich ascites carcinoma cells, and the effect also was confirmed by anatomies of mice [1].
Clinical trial: So far, no clinical study has been conducted.
References:
[1] Yamamoto H, Moriyama K, Jinnouchi H, Yagishita K. Studies on terreic acid. Jpn J Antibiot. 1980 Mar;33(3):320-8.
[2] Kawakami Y, Hartman SE, Kinoshita E, Suzuki H, Kitaura J, Yao L, Inagaki N, Franco A, Hata D, Maeda-Yamamoto M, Fukamachi H, Nagai H, Kawakami T. Terreic acid, a quinone epoxide inhibitor of Bruton's tyrosine kinase. Proc Natl Acad Sci U S A. 1999 Mar 2;96(5):2227-32.
- Vanillic acid
Catalog No.:BCN6105
CAS No.:121-34-6
- Vanillin
Catalog No.:BCN2605
CAS No.:121-33-5
- 3-Deazaneplanocin A (DZNep) hydrochloride
Catalog No.:BCC3604
CAS No.:120964-45-6
- FPL 64176
Catalog No.:BCC7050
CAS No.:120934-96-5
- Isoliquiritin apioside
Catalog No.:BCN2914
CAS No.:120926-46-7
- PF-03394197(Oclacitinib)
Catalog No.:BCC6474
CAS No.:1208319-26-9
- N6022
Catalog No.:BCC4127
CAS No.:1208315-24-5
- Ketone Ester
Catalog No.:BCC1677
CAS No.:1208313-97-6
- VU 0365114
Catalog No.:BCC6164
CAS No.:1208222-39-2
- CaMKII-IN-1
Catalog No.:BCC5530
CAS No.:1208123-85-6
- Quassidine B
Catalog No.:BCN7022
CAS No.:1207862-37-0
- Gynosaponin I
Catalog No.:BCN4078
CAS No.:1207861-69-5
- Benzethonium Chloride
Catalog No.:BCC4635
CAS No.:121-54-0
- N-Acetylsulfanilyl chloride
Catalog No.:BCC9084
CAS No.:121-60-8
- 2-Amino-5-nitrothiazole
Catalog No.:BCC8538
CAS No.:121-66-4
- Propyl gallate
Catalog No.:BCN8431
CAS No.:121-79-9
- 3'-Nitroacetophenone
Catalog No.:BCN2256
CAS No.:121-89-1
- ST 1936 oxalate
Catalog No.:BCC7919
CAS No.:1210-81-7
- N-Acetyl-5-Hydroxytryptamine
Catalog No.:BCC9080
CAS No.:1210-83-9
- JZL 195
Catalog No.:BCC7966
CAS No.:1210004-12-8
- Secretin (rat)
Catalog No.:BCC5848
CAS No.:121028-49-7
- Nelarabine
Catalog No.:BCC1072
CAS No.:121032-29-9
- IEM 1460
Catalog No.:BCC7135
CAS No.:121034-89-7
- PF-04971729
Catalog No.:BCC1852
CAS No.:1210344-57-2
Molecular genetic characterization of terreic acid pathway in Aspergillus terreus.[Pubmed:25265334]
Org Lett. 2014 Oct 17;16(20):5250-3.
Terreic acid is a natural product derived from 6-methylsalicylic acid (6-MSA). A compact gene cluster for its biosynthesis was characterized. Isolation of the intermediates and shunt products from the mutant strains, combined with bioinformatic analyses, allowed for the proposition of a biosynthetic pathway for terreic acid.
Escherichia coli N-Acetylglucosamine-1-Phosphate-Uridyltransferase/Glucosamine-1-Phosphate-Acetylt ransferase (GlmU) Inhibitory Activity of Terreic Acid Isolated from Aspergillus terreus.[Pubmed:26762501]
J Biomol Screen. 2016 Apr;21(4):342-53.
Secondary metabolite of Aspergillus terreus, terreic acid, is a reported potent antibacterial that was identified more than 60 years ago, but its cellular target(s) are still unknown. Here we screen its activity against the acetyltransferase domain of a bifunctional enzyme, Escherichia coli N-acetylglucosamine-1-phosphate-uridyltransferase/glucosamine-1-phosphate-acetylt ransferase (GlmU). An absorbance-based assay was used to screen terreic acid against the acetyltransferase activity of E. coli GlmU. Terreic acid was found to inhibit the acetyltransferase domain of E. coli GlmU with an IC50 of 44.24 +/- 1.85 microM. Mode of inhibition studies revealed that terreic acid was competitive with AcCoA and uncompetitive with GlcN-1-P. It also exhibited concentration-dependent killing of E. coli ATCC 25922 up to 4x minimum inhibitory concentration and inhibited the growth of biofilms generated by E. coli. Characterization of resistant mutants established mutation in the acetyltransferase domain of GlmU. Terreic acid was also found to be metabolically stable in the in vitro incubations with rat liver microsome in the presence of a NADPH regenerating system. The studies reported here suggest that terreic acid is a potent antimicrobial agent and support that E. coli GlmU acetyltransferase is a molecular target of terreic acid, resulting in its antibacterial activity.
Differential antibacterial properties of the MurA inhibitors terreic acid and fosfomycin.[Pubmed:23686727]
J Basic Microbiol. 2014 Apr;54(4):322-6.
Terreic acid is a metabolite with antibiotic properties produced by the fungus Aspergillus terreus, but its cellular target remains unknown. We recently reported that terreic acid inactivates the bacterial cell wall biosynthetic enzyme MurA in vitro by covalent reaction with residue Cys115 in a similar manner as the MurA-specific antibiotic fosfomycin. To address if terreic acid also targets MurA in vivo, we conducted antibacterial studies using selected E. coli strains in parallel with fosfomycin. While overexpression of MurA conferred resistance to fosfomycin, it did not protect cells treated with terreic acid. Furthermore, flow cytometry revealed that the antibiotic action of terreic acid appears to be primarily bacteriostatic, as opposed to the bactericidal action observed for fosfomycin. Combined, the data suggest that MurA is not the molecular target of terreic acid and that the antibiotic activity of terreic acid proceeds through a different mechanism of action. The methodology applied here provides a reliable and convenient tool to rapidly assess the potential of newly discovered in vitro inhibitors to target residue Cys115 of MurA in the cell.
The fungal product terreic acid is a covalent inhibitor of the bacterial cell wall biosynthetic enzyme UDP-N-acetylglucosamine 1-carboxyvinyltransferase (MurA) .[Pubmed:20392080]
Biochemistry. 2010 May 18;49(19):4276-82.
Terreic acid is a metabolite with antibiotic properties produced by the fungus Aspergillus terreus. We found that terreic acid is a covalent inhibitor of the bacterial cell wall biosynthetic enzyme MurA from Enterobacter cloacae and Escherichia coli in vitro. The crystal structure of the MurA dead-end complex with terreic acid revealed that the quinine ring is covalently attached to the thiol group of Cys115, the molecular target of the antibiotic fosfomycin. Kinetic characterization established that the inactivation requires the presence of substrate UNAG (UDP-N-acetylglucosamine), proceeding with an inactivation rate constant k(inact) of 130 M(-1) s(-1). Although the mechanisms of inactivation are similar, fosfomycin is approximately 50 times more potent than terreic acid, and the structural consequences of covalent modification by these two inhibitors are fundamentally different. The MurA-fosfomycin complex exists in the closed enzyme conformation, with the Cys115-fosfomycin adduct buried in the active site. In contrast, the dead-end complex with terreic acid is open, is free of UNAG, and has the Cys115-terreic acid adduct solvent-exposed. It appears that terreic acid reacts with Cys115 in the closed, binary state of the enzyme, but that the resulting Cys115-terreic acid adduct imposes steric clashes in the active site. As a consequence, the loop containing Cys115 rearranges, the enzyme opens, and UNAG is released. The differential kinetic and structural characteristics of MurA inactivation by terreic acid and fosfomycin reflect the importance of noncovalent binding potential, even for covalent inhibitors, in ensuring inactivation efficiency and specificity.
Terreic acid, a quinone epoxide inhibitor of Bruton's tyrosine kinase.[Pubmed:10051623]
Proc Natl Acad Sci U S A. 1999 Mar 2;96(5):2227-32.
Bruton's tyrosine kinase (Btk) plays pivotal roles in mast cell activation as well as in B cell development. Btk mutations lead to severe impairments in proinflammatory cytokine production induced by cross-linking of high-affinity IgE receptor on mast cells. By using an in vitro assay to measure the activity that blocks the interaction between protein kinase C and the pleckstrin homology domain of Btk, terreic acid (TA) was identified and characterized in this study. This quinone epoxide specifically inhibited the enzymatic activity of Btk in mast cells and cell-free assays. TA faithfully recapitulated the phenotypic defects of btk mutant mast cells in high-affinity IgE receptor-stimulated wild-type mast cells without affecting the enzymatic activities and expressions of many other signaling molecules, including those of protein kinase C. Therefore, this study confirmed the important roles of Btk in mast cell functions and showed the usefulness of TA in probing into the functions of Btk in mast cells and other immune cell systems. Another insight obtained from this study is that the screening method used to identify TA is a useful approach to finding more efficacious Btk inhibitors.
In vivo and in vitro studies on the binding nature of terreic acid with macromolecules such as protein and nucleic acids.[Pubmed:7080093]
Toxicol Lett. 1982 Feb;10(2-3):249-53.
The binding of terreic acid to macromolecules such as nucleic acids and protein was examined. In vivo studies using [14C]terreic acid showed that radioactivity was incorporated into the protein and nucleic acid fractions of the liver mice. In vitro experiments with human serum showed that [14C]terreic acid bound to the albumin component and spectral studies indicated that terreic acid also combined with hepatic DNA.
Studies on terreic acid.[Pubmed:7190624]
Jpn J Antibiot. 1980 Mar;33(3):320-8.
It was found that Aspergillus sp. No. Y-8980 which was isolated from a soil sample collected at Yoron Island in Kagoshima Prefecture belonged to Aspergillus terreus group by morphological observation. The active substance produced by the strain was obtained with a high yield in sucrose-yeast extract medium and extracted by chloroform, ethyl acetate and n-butanol at pH 2.4 approximately 2.6 from the culture broth. The substance was crystallized from chloroform and ethyl acetate after charcoal treatment of the crude crystal. From various physico-chemical properties, it was found that the substance was identical to terreic acid. Terreic acid showed MICs of 25 approximately 100 mcg/ml, 12.5 mcg/ml and 50 mcg/ml against Gram-positive and Gram-negative bacteria, Xanthomonas oryzae and Xanthomonas citri, respectively, but it did not control Pseudomonas, fungi and yeast. The LD50 was 75 mg/kg i.p. and i.v. in mice. With regards to the anti-tumor effect, the morphological degeneration on HeLa cells (human carcinoma cells) was observed in the concentrations of more than 6.25 mcg/ml of terreic acid. An increase of body weight of mice caused by Ehrlich ascites carcinoma cells was not definitely observed by the daily administration of 150 mcg of terreic acid per mouse for 8 consecutive days. Above showed the enough survival effect in dd mice implanted with Ehrlich ascites carcinoma cells, and the effect also was demonstrated by anatomies of mice.


