Morus alba
Morus alba
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
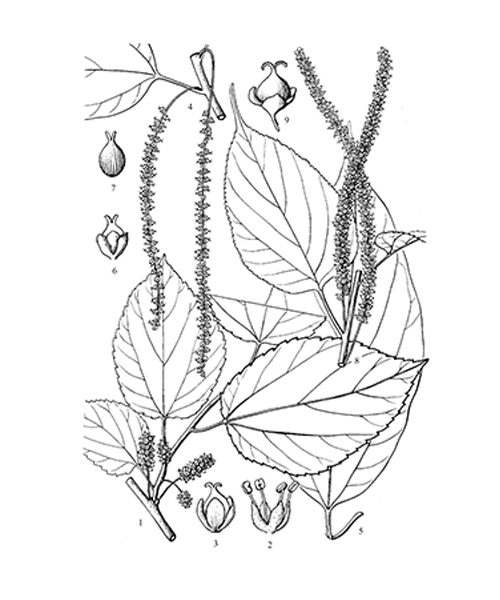
Natural products/compounds from Morus alba
- Cat.No. Product Name CAS Number COA
-
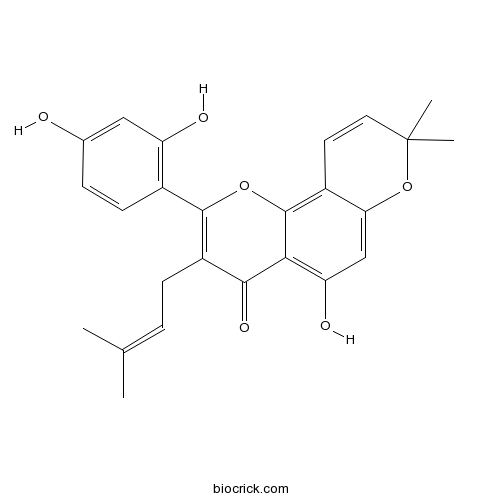
BCN8844
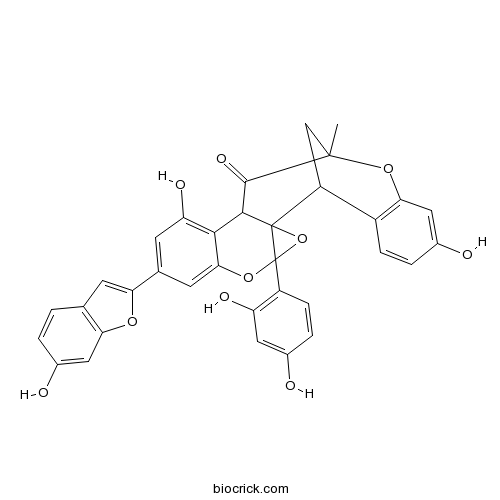
Mulberrofuran Q101383-35-1
Instructions
-
BCN6343
Mulberroside A102841-42-9
Instructions

-
BCN6344
Mulberroside C102841-43-0
Instructions

-
BCN3289
Moracin P102841-46-3
Instructions

-
BCN6303
Betaine107-43-7
Instructions

-
BCN5890
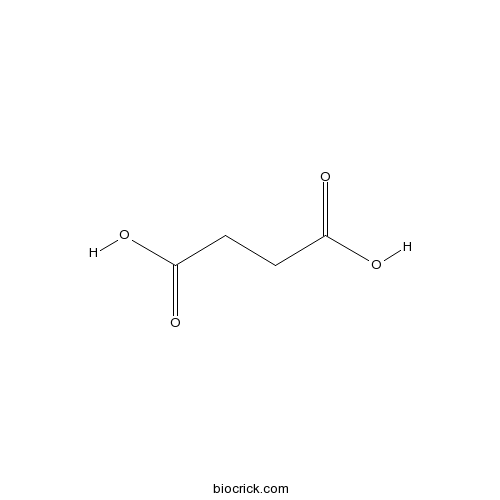
Succinic acid110-15-6
Instructions
-
BCN4004
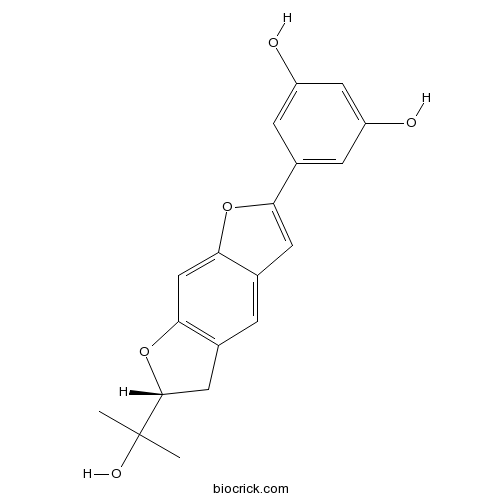
Moracin O123702-97-6
Instructions
-
BCN2596
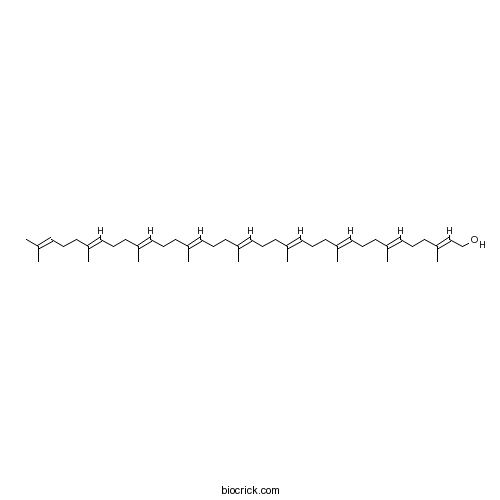
Solanesol13190-97-1
Instructions
-
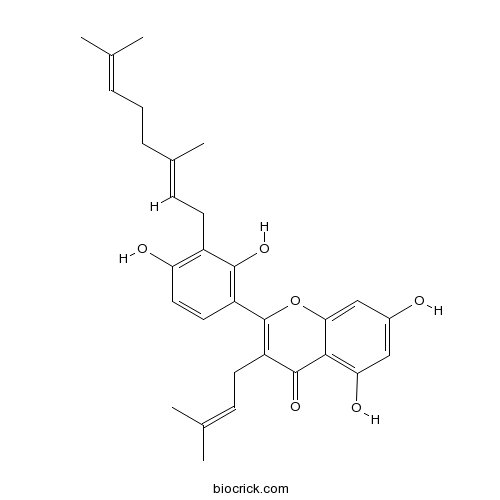
BCN1583
3'-Geranyl-3-prenyl-2',4',5,7-tetrahydroxyflavone1334309-44-2
Instructions
-
BCC8957
Episyringaresinol 4'-O-β-D-glncopyranoside137038-13-2
Instructions

-
BCN1566
Oxyresveratrol 3'-O-beta-D-glucopyranoside144525-40-6
Instructions

-
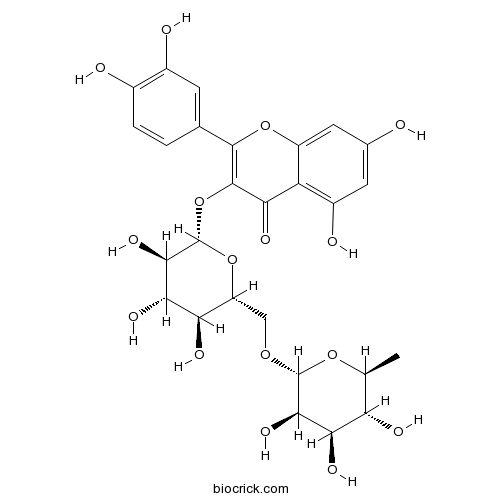
BCN1684
Rutin153-18-4
Instructions
-
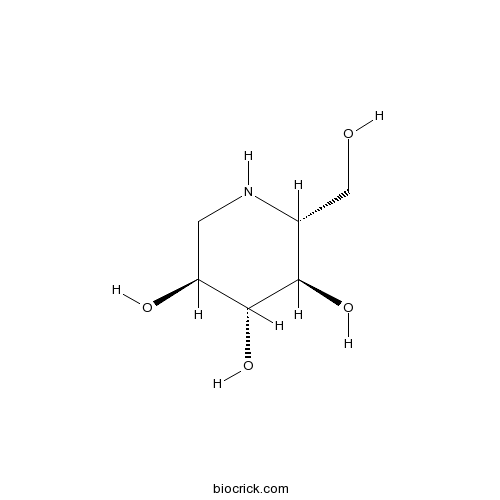
BCN1032
1-Deoxynojirimycin19130-96-2
Instructions
-
BCN2908
Mulberroside F193483-95-3
Instructions
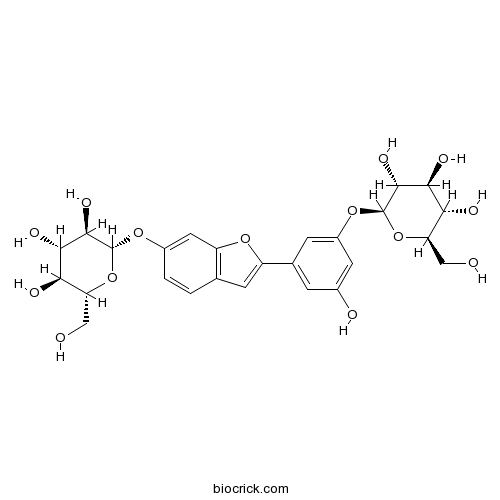
-
BCN7840
Multicaulisin286461-76-5
Instructions

-
BCN5201
Oxyresveratrol29700-22-9
Instructions

-
BCN5906
Chlorogenic acid327-97-9
Instructions
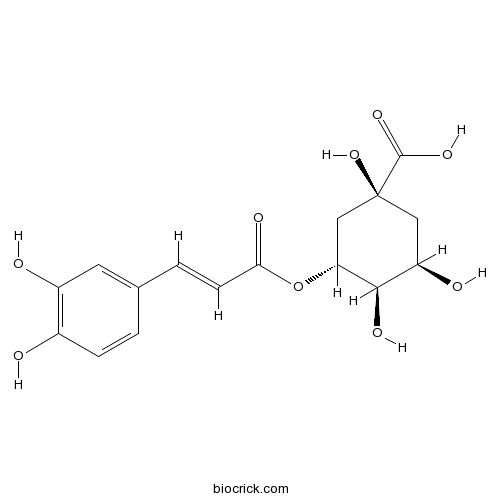
-
BCN3692
Sanggenol L329319-20-2
Instructions

-
BCN1448
Oxyresveratrol 2-O-beta-D-glucopyranoside392274-22-5
Instructions
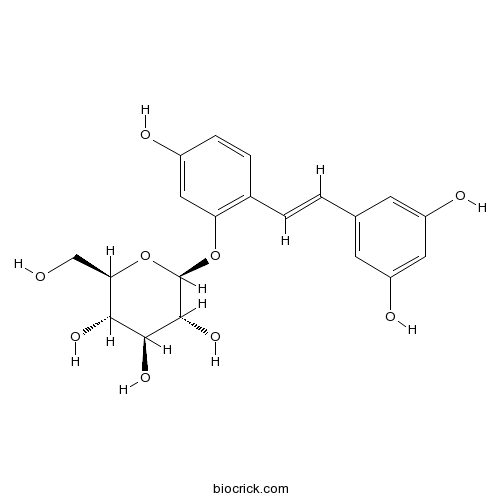
-
BCN5549
Astragalin480-10-4
Instructions

-
BCN1028
Morin480-16-0
Instructions

-
BCN5607
Resveratrol501-36-0
Instructions

-
BCN5701
Scopolin531-44-2
Instructions
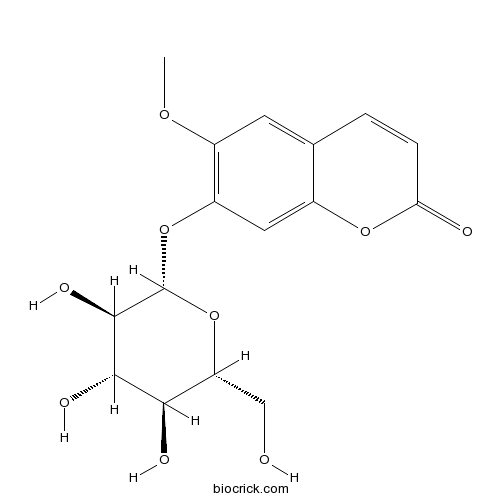
-
BCN3820
Stearic Acid57-11-4
Instructions

-
BCN4165
Morusin62596-29-6
Instructions
-
BCN2944
Kuwanon A62949-77-3
Instructions

-
BCN4167
Mulberrin62949-79-5
Instructions

-
BCN4168
Morusinol62949-93-3
Instructions

-
BCN3287
Kuwanon E68401-05-8
Instructions

-
BCC4109
Salicylic acid69-72-7
Instructions

-
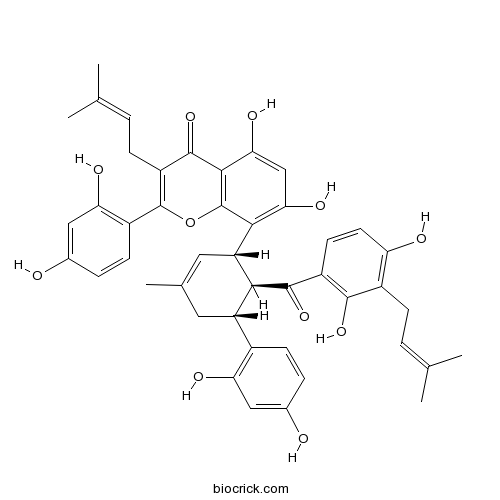
BCN2945
Kuwanon H76472-87-2
Instructions
-
BCN6350
Sanggenone C80651-76-9
Instructions

-
BCN1194
Sanggenone D81422-93-7
Instructions

-
BCN2946
Sanggenone H86450-80-8
Instructions
-
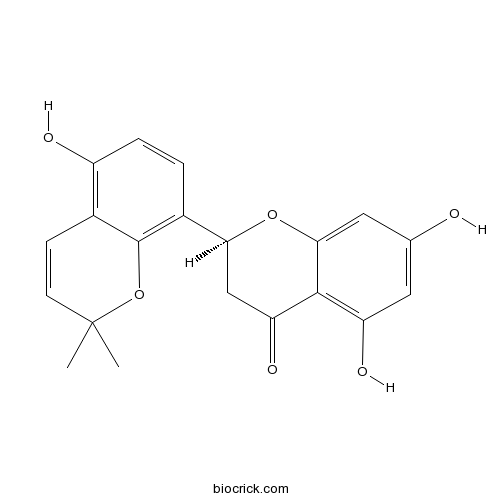
BCN3693
Mulberrofuran G87085-00-5
Instructions

-
BCN4477
Umbelliferone93-35-6
Instructions

Chalcone derivatives from the root bark of Morus alba L. act as inhibitors of PTP1B and α-glucosidase.[Pubmed: 30103164]
None
Molecular characterization of a geranyl diphosphate-specific prenyltransferase catalyzing stilbenoid prenylation from Morus alba.[Pubmed: 30020500]
Pharmaceutically active compounds from medical plants are attractive as a major source for new drug development. Prenylated stilbenoids with increased lipophilicity are valuable secondary metabolites, which possess a wide range of biological activities. So far, many prenylated stilbenoids have been isolated from Morus alba but the enzyme responsible for the crucial prenyl-modification remains unknown. In the present study, a stilbenoid-specific prenyltransferase (PT), termed Morus alba oxyresveratrol geranyltransferase (MaOGT), was identified and functionally characterized in vitro. MaOGT recognized oxyresveratrol and geranyl diphosphate (GPP) as natural substrates, and catalyzed oxyresveratrol prenylation. Our results indicated that MaOGT shared common features with other aromatic PTs, e.g. multiple transmembrane regions, conserved functional domains, and targeting to plant plastids. This distinct PT represents the first stilbenoid-specific PT accepting GPP as a natural prenyl donor, and could help identify additional functionally varied PTs in moraceous plants. Furthermore, MaOGT might be applied for high-efficiency and large-scale prenylation of oxyresveratrol to produce bioactive compounds for potential therapeutic applications.
Comparative study of chemical composition and active components against α-glucosidase of various medicinal parts of Morus alba L.[Pubmed: 29975423]
Morus alba L has long been used as fodder and as a traditional medicine. Various parts of M. alba (Cortex mori, Ramulus mori, Folium mori and Fructus mori) have various bioactivities, however, most current evidence focused on anti-diabetic properties. In spite of their wide use, few studies compared the chemical composition and active components against α-glucosidase of the various medicinal parts of M. alba. In this study, we developed an HPLC method for simultaneous quality control and discrimination of Cortex mori, Ramulus mori, Folium mori and Fructus mori using thirteen marker compounds. We found that quercetin, morin, kuwanon G, sanggenon C, morusin, mulberroside A and rutin were chemically distinct among the various medicinal parts of M. alba. A spectrum-effect relationship method was established to compare α-glucosidase inhibitory activity of various batches of samples to determine the activity of the primary active components against α-glucosidase. Taken together with molecular docking data, we found that prenylated flavonoids (morin, sanggenon C, kuwanon G and morusin), flavonols (kaempferol, quercetin, rutin and isoquercitrin) and alkaloids (1-deoxynojirimycin) were small molecule α-glucosidase inhibitory ingredients. In conclusion, we laid a solid foundation for effective substance identification in various parts of M. alba, and simultaneously provided a basis for their quality control.
Morus alba Leaf Bioactives Modulate Peroxisome Proliferator Activated Receptor γ in the Kidney of Diabetic Rat and Impart Beneficial Effect.[Pubmed: 29969905]
Peroxisome proliferator activated receptor gamma (PPARγ) is a ligand-activated nuclear receptor that can be activated or repressed by several exogenous and endogenous ligands and acts by modulating genes that regulate lipid, glucose, and insulin homeostasis. In kidney, PPARγ is involved in normal kidney development and other physiological functions. In our earlier report, we showed that feeding Morus alba leaves to experimental diabetic rats ameliorated diabetic nephropathy and significantly decreased microalbuminuria. In this paper, we have attempted to look into the molecular mechanism involving PPARγ modulation by mulberry leaf bioactive compounds by in vitro and in vivo methods and its impact on key inflammatory markers. In vitro assay by TR-FRET suggested that mulberry leaf extracts can serve as a putative modulator of PPARγ. High glucose conditions in vitro and in vivo increased PPARγ levels, which were ameliorated by mulberry leaves or their extracts. Interestingly, PPARγ was significantly phosphorylated at Ser112 by upstream kinases ERK42/44 in kidney of diabetic animals on feeding mulberry leaves. In vitro studies using MDCK cell line revealed that increased Ser112 phosphorylation was observed when cells were treated with bound phenolic acid rich extract but not with free phenolic acid rich extracts. HPLC analysis and bioassay-guided activity revealed that coumaric acid was the bioactive molecule within bound phenolic acid rich extract that was responsible for increased ERK42/44-mediated phosphorylation at Ser112. Furthermore, mulberry leaf bioactive compounds showed beneficial effect on the tested inflammatory markers.
Efficient production of succinic acid from herbal extraction residue hydrolysate.[Pubmed: 29935453]
In this study, six different herbal-extraction residues were evaluated for succinic acid production in terms of chemical composition before and after dilute acid pretreatment (DAP) and sugar release performance. Chemical composition showed that pretreated residues of Glycyrrhiza uralensis Fisch (GUR) and Morus alba L. (MAR) had the highest cellulose content, 50% and 52%, respectively. Higher concentrations of free sugars (71.6 g/L total sugar) and higher hydrolysis yield (92%) were both obtained under 40 FPU/g DM at 10% solid loading for GUR. Using scanning electron microscopy (SEM), GUR was found to show a less compact structure due to process of extraction. Specifically, the fibers in pretreated GUR were coarse and disordered compared with that of GUR indicated by SEM. Finally, 65 g/L succinic acid was produced with a higher yield of 0.89 g/g total sugar or 0.49 g/g GUR. Our results illustrate the potential of GUR for succinic acid production.
Sipi soup inhibits cancer‑associated fibroblast activation and the inflammatory process by downregulating long non‑coding RNA HIPK1‑AS.[Pubmed: 29901171]
Sipi soup (SPS), the aqueous extract derived from the root bark of Sophora japonical L, Salix babylonica L., Morus alba L., as well as Amygdalus davidiana (Carr.) C. de Vos, is a traditional Chinese medicine frequently used to prevent and treat infection and inflammation. However, the role of SPS in cancer‑associated fibroblasts (CAFs) require further investigation. In the present study, the effects of SPS on fibroblast inactivation and the underlying mechanism were investigated. Reverse transcription‑quantitative polymerase chain reaction was used to analyze the mRNA expression levels of fibroblast activation protein (FAP), interleukin (IL)‑6, α‑smooth muscle actin (α‑SMA) and programmed cell death 4 (PDCD4). Flow cytometry was used to evaluate cell apoptosis. Immunofluorescence was used to determine the number of activated fibroblasts. The present study reported that SPS treatment did not affect the proliferative apoptotic potential of fibroblasts. Treatment with HeLa cell culture medium (CM) induced a significant increase in the expression levels of FAP, IL‑6 and α‑SMA, but reduced the expression of PDCD4. SPS reversed the effects of HeLa CM on the expression of these genes. Analysis with a long non‑coding (lnc)RNA array of numerous differentially expressed lncRNAs revealed that the expression levels of the lncRNA homeodomain‑interacting protein kinase 1 antisense RNA (HIPK1‑AS) were increased in cervicitis tissues and cervical squamous cell carcinoma tissues compared with in normal cervical tissues. HIPK1‑AS expression levels were upregulated in response to HeLa CM, but were decreased under SPS treatment. The downregulation of HIPK1‑AS expression via short hairpin RNA abolished the effects of HeLa CM on the expression of inflammation‑associated genes. The findings of the present study suggested that SPS may prevent the progression of cervical cancer by inhibiting the activation of CAF and the inflammatory process by reducing HIPK1‑AS expression.


