Schisandra chinensis
Schisandra chinensis
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Schisandra chinensis
- Cat.No. Product Name CAS Number COA
-
BCN2674
Negsehisandrin G1023744-69-5
Instructions
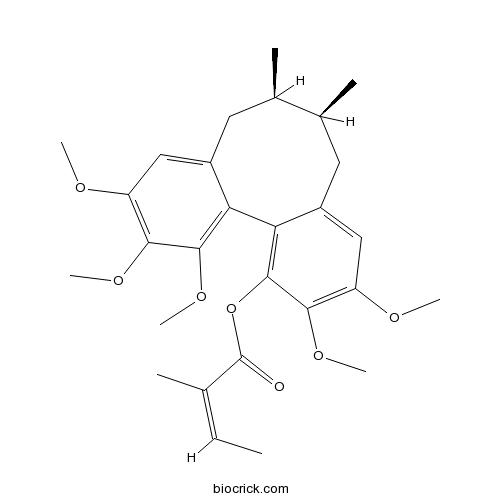
-
BCN5362
Anwulignan107534-93-0
Instructions
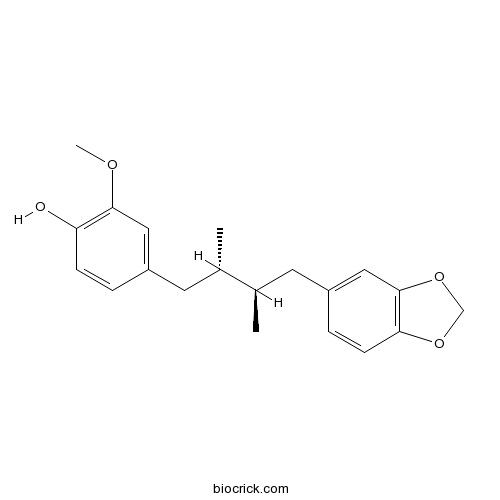
-
BCN5808
Wulignan A1117047-76-4
Instructions

-
BCN3315
Schisanwilsonin H1181216-83-0
Instructions
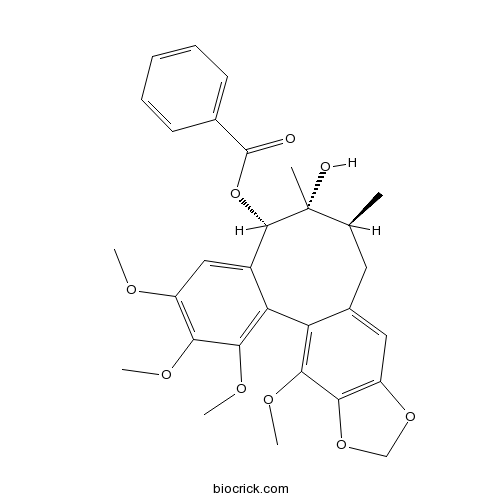
-
BCN5548
Schisanwilsonin I1181216-84-1
Instructions
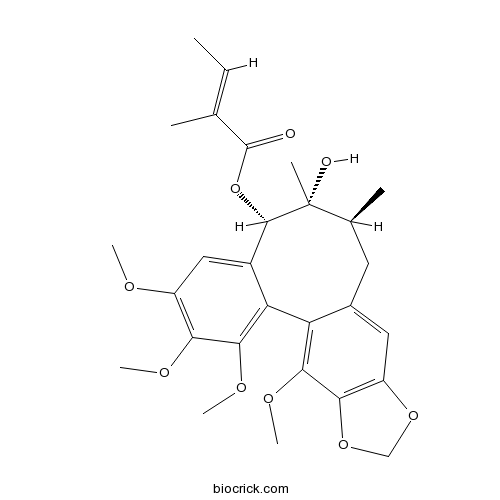
-
BCN1521
rel-(8R,8'R)-dimethyl-(7S,7'R)-bis(3,4-methylenedioxyphenyl)tetrahydro-furan178740-32-4
Instructions
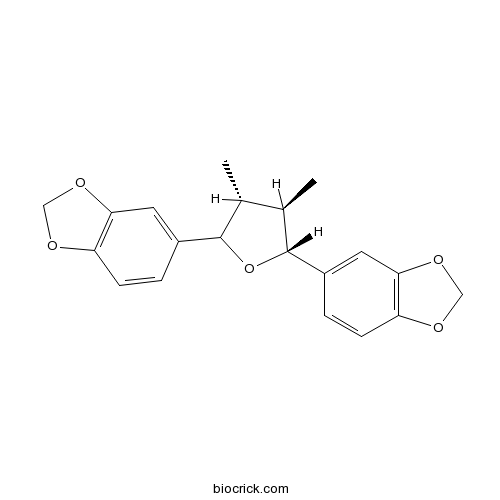
-
BCN7847
Chamigrenal19912-84-6
Instructions

-
BCN1240
Dehydrodiisoeugenol2680-81-1
Instructions
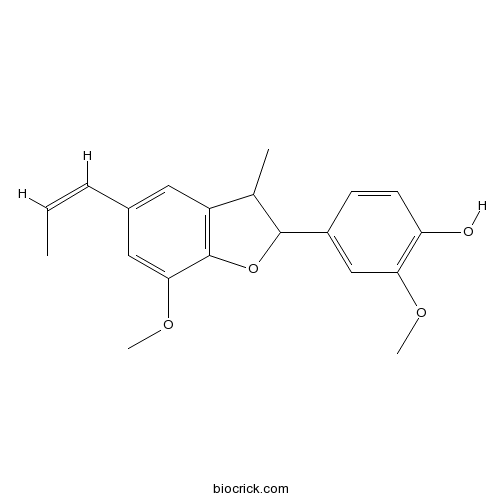
-
BCN5979
Caffeic acid331-39-5
Instructions
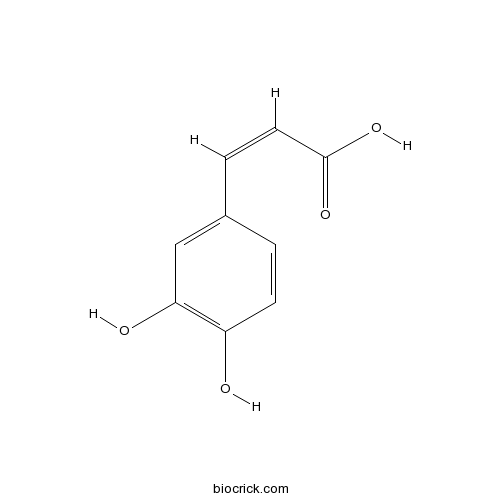
-
BCN5590
Daidzein486-66-8
Instructions

-
BCN8390
Myristic acid544-63-8
Instructions

-
BCN2851
Quercetin 3-O-beta-D-xylopyranoside549-32-6
Instructions

-
BCN5794
Gomisin A58546-54-6
Instructions
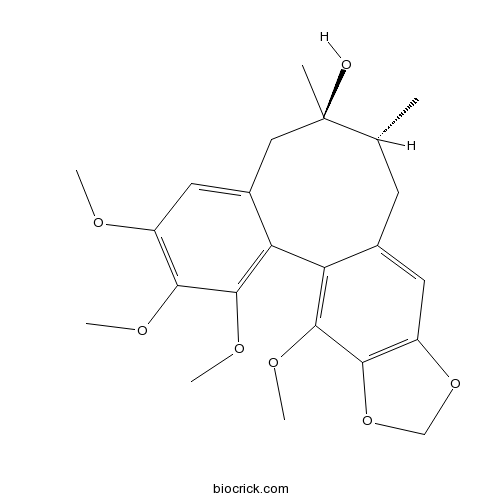
-
BCN1023
Schisantherin B58546-55-7
Instructions
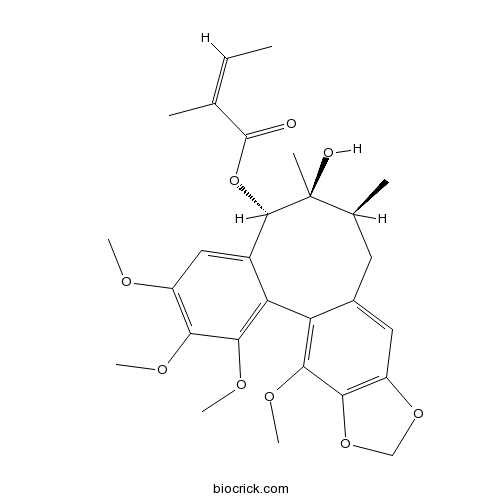
-
BCN1024
Schisantherin A58546-56-8
Instructions
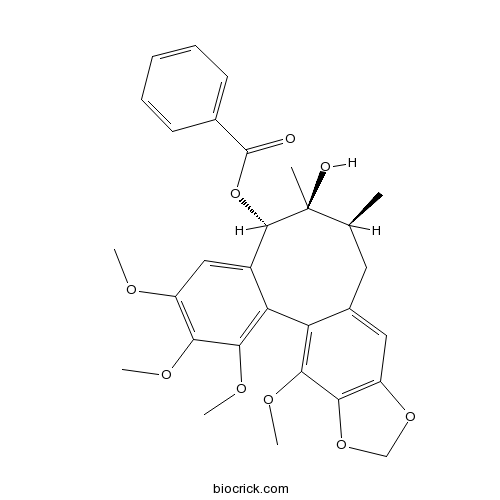
-
BCN2268
Gomisin D60546-10-3
Instructions
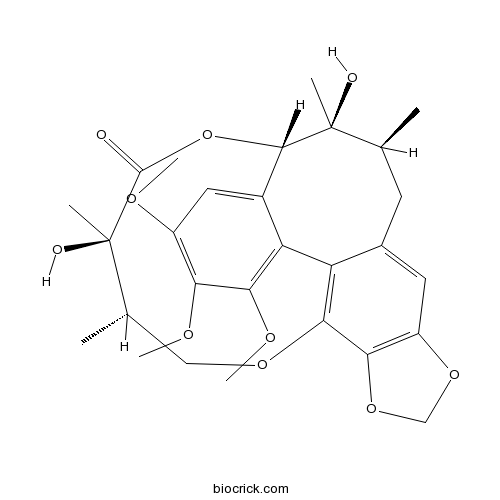
-
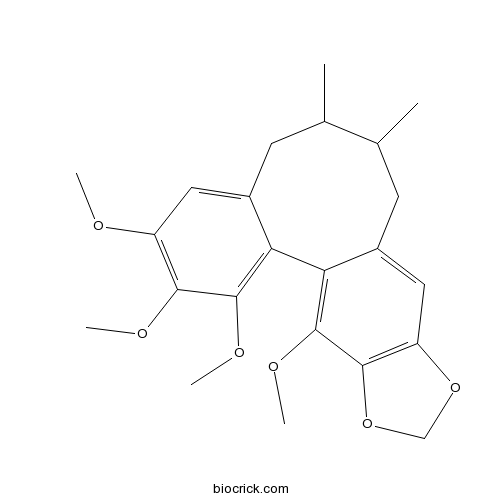
BCN1022
Schizandrin B61281-37-6
Instructions
-
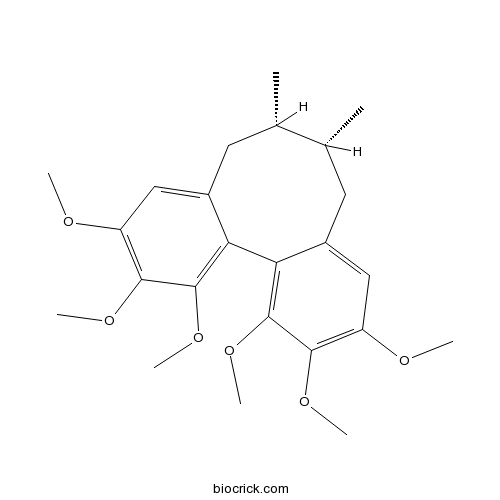
BCN1021
Schizandrin A61281-38-7
Instructions
-
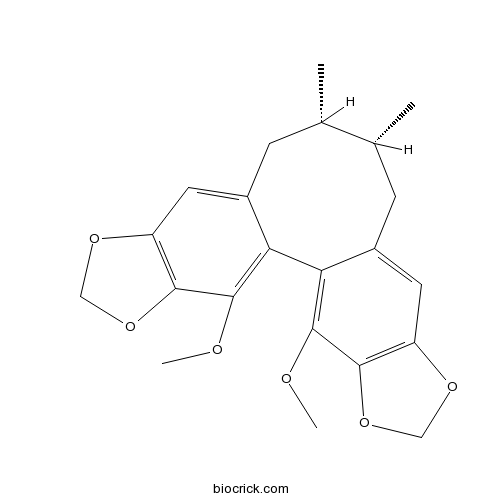
BCN1198
Schisandrin C61301-33-5
Instructions
-
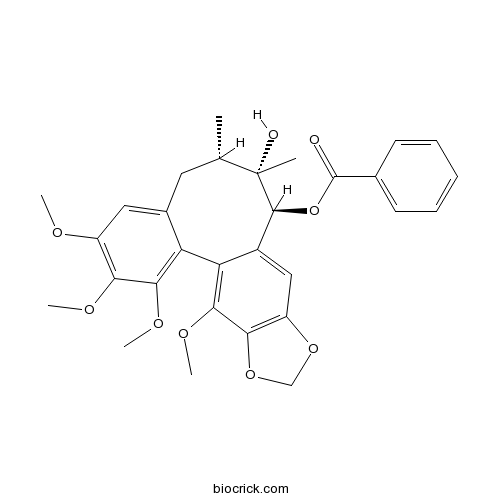
BCN2269
Gomisin G62956-48-3
Instructions
-
BCN6766
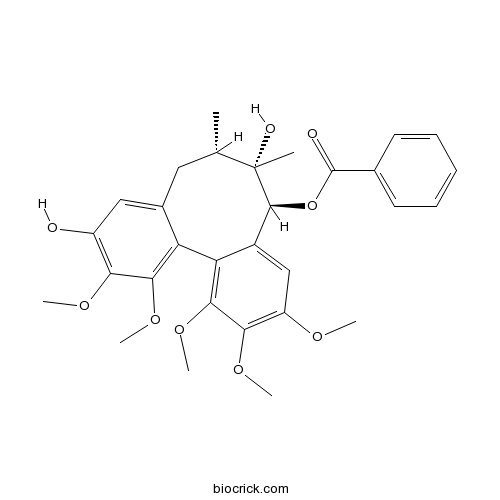
Schisantherin E64917-83-5
Instructions
-
BCN3902
Gomisin H66056-20-0
Instructions
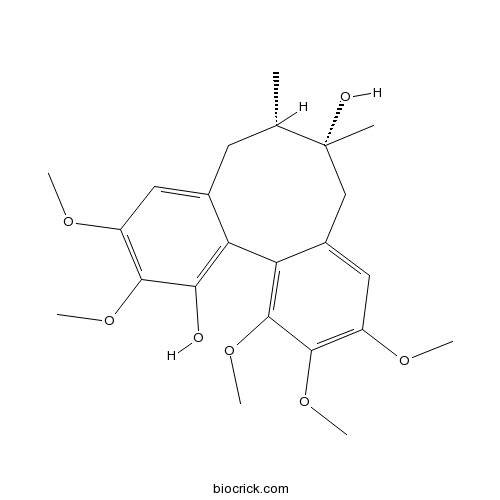
-
BCN2843
Angeloylgomisin H66056-22-2
Instructions
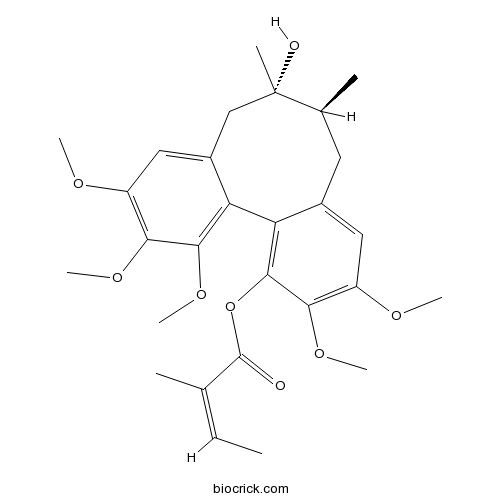
-
BCN2270
Gomisin J66280-25-9
Instructions
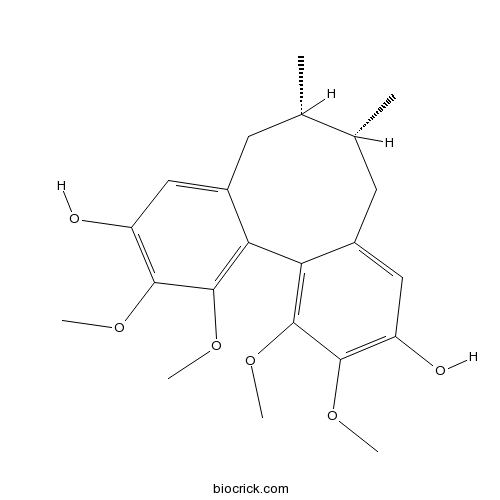
-
BCN2508
Schisanhenol69363-14-0
Instructions

-
BCN2875
Gomisin O72960-22-6
Instructions
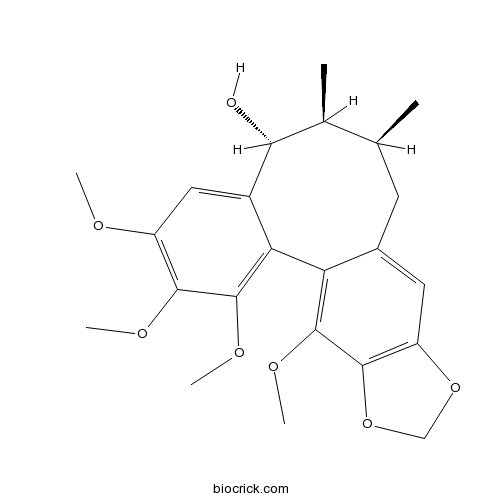
-
BCN5815
Schisandrin A7432-28-2
Instructions

-
BCN1029
D-(-)-Quinic acid77-95-2
Instructions
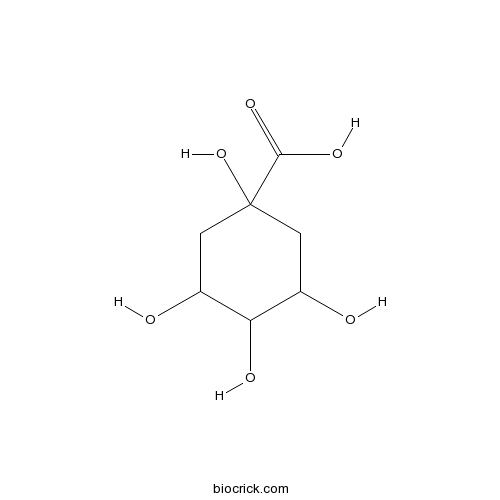
-
BCN7818
Chicanine78919-28-5
Instructions
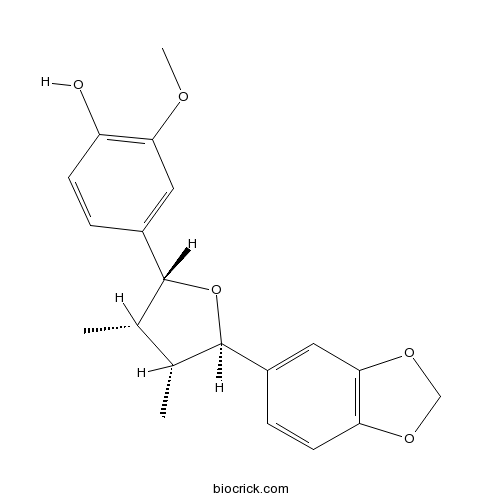
-
BCX0642
Isokadsuranin82467-52-5
Instructions

-
BCN7361
Angeloylgomisin O83864-69-1
Instructions
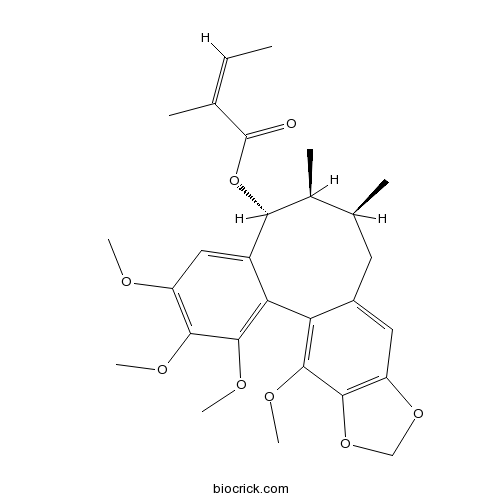
-
BCN2863
(-)-Holostyligone887501-28-2
Instructions
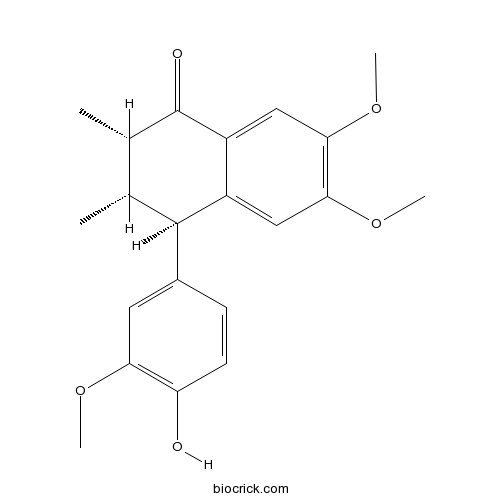
-
BCN3316
Schisandrone98619-25-1
Instructions
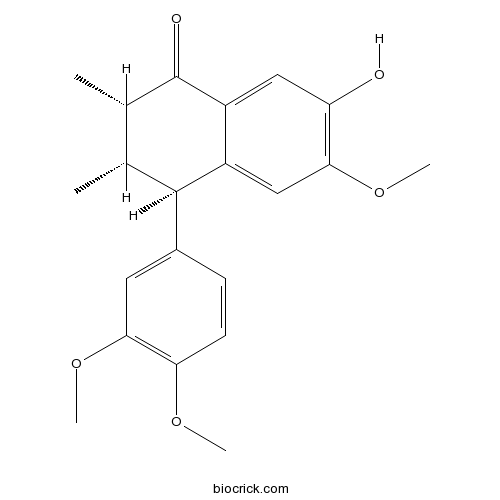
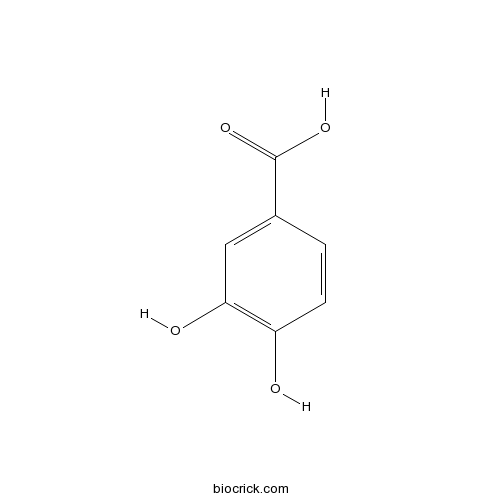
-
BCN4537
3,4-Dihydroxybenzoic acid99-50-3
Instructions
[Optimization of Aqueous Two-Phase Extraction of Polysaccharide from Schisandra chinensis Using Response Surface Methodology with Box-Behnken Design].[Pubmed: 30091354]
To optimize the extraction condition of polysaccharide from Schisandra chinensis. Aqueous two-phase extraction( ATPE) method was used, based on Box-Behnken design with Response surface methodology( BBD-RSM).
Protective effects of Schisandrin on high glucose-induced changes of RhoA and eNOS activity in human umbilical vein endothelial cells.[Pubmed: 30031586]
Schisandrin, derived from the Chinese medicinal herb Schisandra chinensis, has been found to confer protective effects on circulation systems. But the underlying molecular mechanisms remain unclear. The aim of this study was to investigate the effects of a high level of glucose on RhoA and eNOS activity in human umbilical vein endothelial cells(HUVECs) and how Schisandrin plays a role in mediating these effects. To find the optimal treatment time, HUVECs were cultured at a high glucose concentration (30 mM) for different lengths of time (0, 12, 24, and 48 h). Subsequently, the cells were randomized into five groups: a normal group, a high glucose group, and three high glucose groups that were given different doses (5, 10, and 20 μM) of Schisandrin. The cells were pretreated with Schisandrin for 24 h before stimulation with high glucose. The morphology of HUVECs in the various groups was assessed under a light microscope. Immunocytochemical staining was used to detect the level of p-MYPT1 expression. The levels of RhoA activity were determined using the RhoA Activation Assay Biochem Kit. The levels of eNOS activity were examined using a nitrate reduction test. The results showed that in the high glucose group, the activity of RhoA was increased and the activity of eNOS was reduced, thus decreasing the secretion of NO. However, after pretreatment with Schisandrin (10, 20 μM), the activity of RhoA was inhibited and the activity of eNOS increased, which led to an increase in NO production compared with the high glucose group. There was no evident difference between the 5 μM Schisandrin group and the high glucose group. Taken together, these findings indicate that Schisandrin can improve the function of endothelial cells by lowering the activity of RhoA/Rho kinase and raising both the activity of eNOS and the production of NO.


