Glycyrrhiza uralensis
Glycyrrhiza uralensis
1. The products in our compound library are selected from thousands of unique natural products; 2. It has the characteristics of diverse structure, diverse sources and wide coverage of activities; 3. Provide information on the activity of products from major journals, patents and research reports around the world, providing theoretical direction and research basis for further research and screening; 4. Free combination according to the type, source, target and disease of natural product; 5. The compound powder is placed in a covered tube and then discharged into a 10 x 10 cryostat; 6. Transport in ice pack or dry ice pack. Please store it at -20 °C as soon as possible after receiving the product, and use it as soon as possible after opening.
Natural products/compounds from Glycyrrhiza uralensis
- Cat.No. Product Name CAS Number COA
-
BCN6312
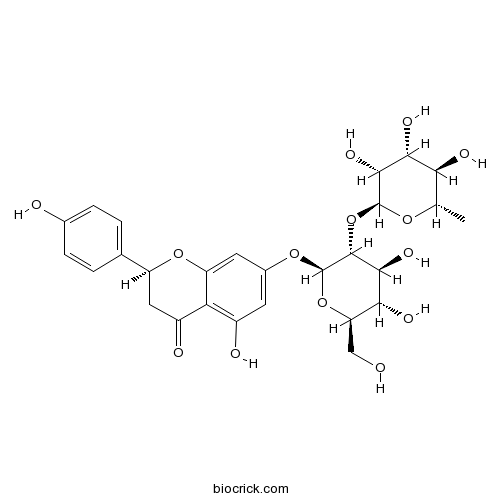
Naringin10236-47-2
Instructions
-
BCN2930
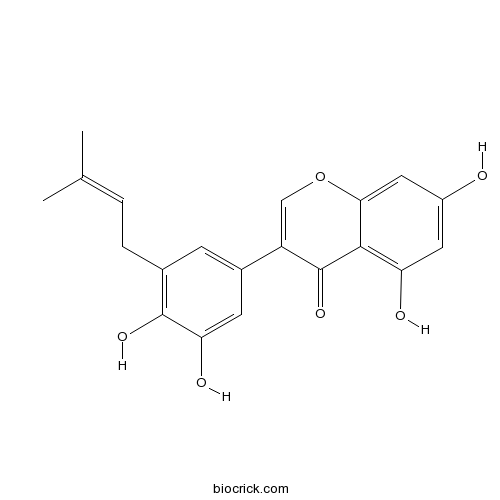
Glycyrrhisoflavone116709-70-7
Instructions
-
BCN2914
Isoliquiritin apioside120926-46-7
Instructions

-
BCN2931
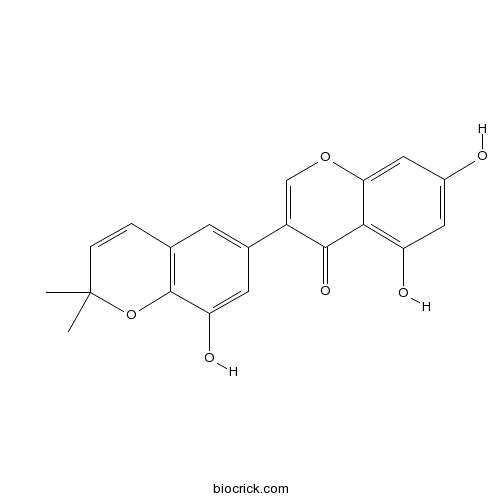
Semilicoisoflavone B129280-33-7
Instructions
-
BCN5941
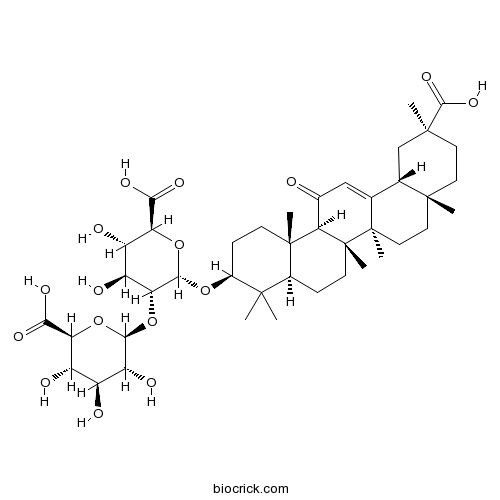
Glycyrrhizic acid1405-86-3
Instructions
-
BCN1684
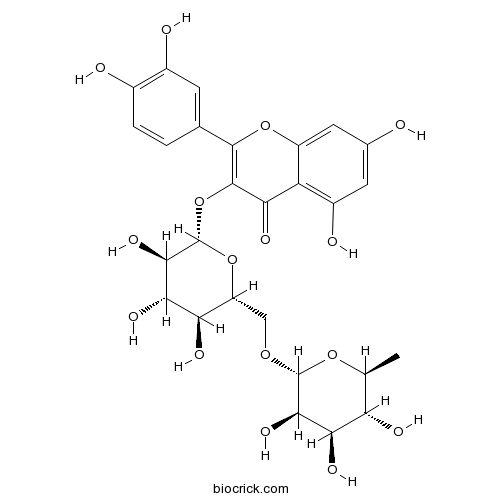
Rutin153-18-4
Instructions
-
BCN5930
Calycosin20575-57-9
Instructions

-
BCN5415
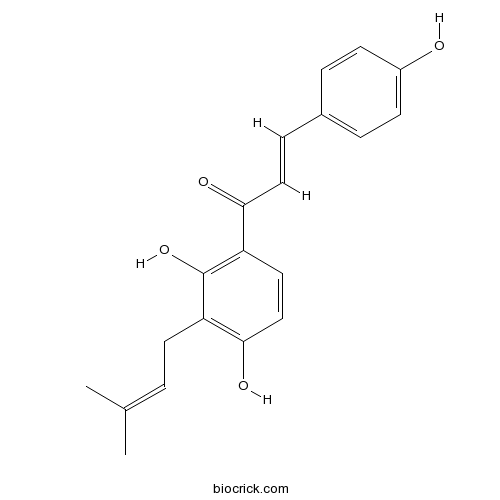
Isobavachalcone20784-50-3
Instructions
-
BCN2907
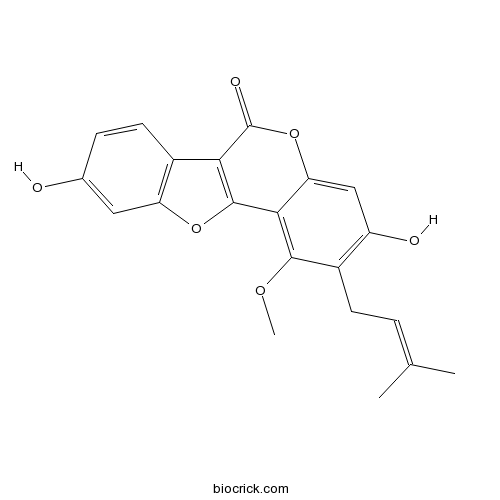
Neoglycyrol23013-84-5
Instructions
-
BCN3013
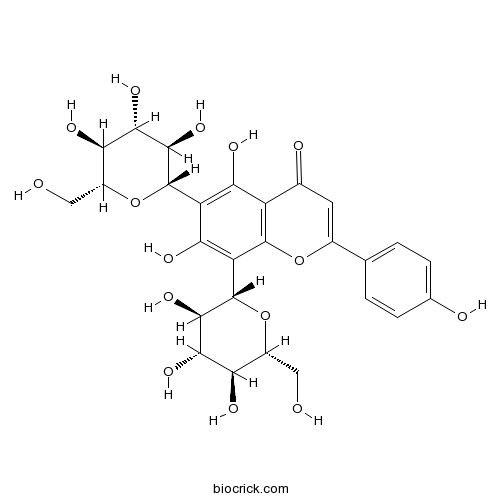
Vicenin -223666-13-9
Instructions
-
BCN6277
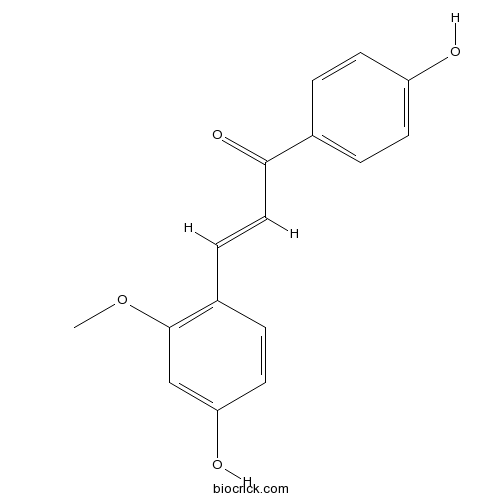
Echinatin34221-41-5
Instructions
-
BCN5895
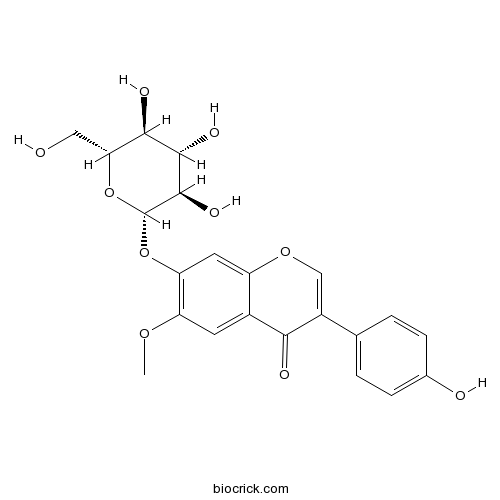
Glycitin40246-10-4
Instructions
-
BCN5488
Genkwanin437-64-9
Instructions

-
BCN5942
Glycyrrhetinic acid471-53-4
Instructions
-
BCN5524
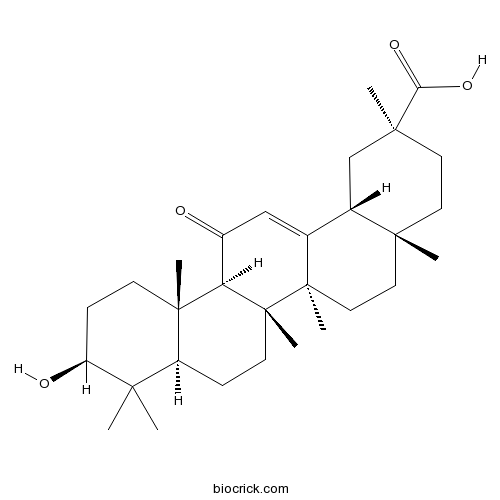
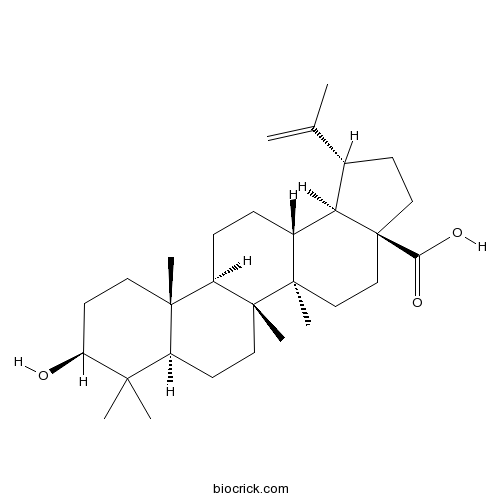
Betulinic acid472-15-1
Instructions
-
BCN5549
Astragalin480-10-4
Instructions

-
BCN5553
Orobol480-23-9
Instructions

-
BCN5556
Pinocembrin480-39-7
Instructions

-
BCN5569
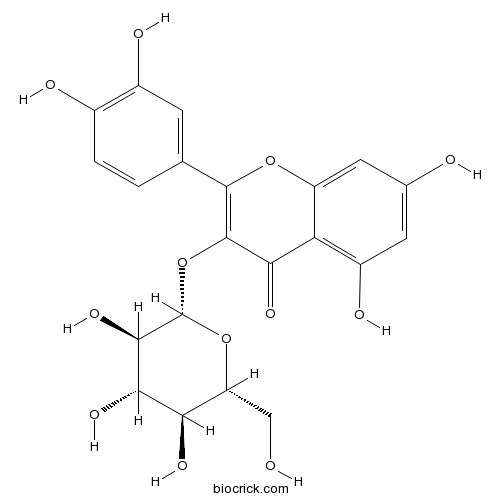
Isoquercitrin482-35-9
Instructions
-
BCN5581
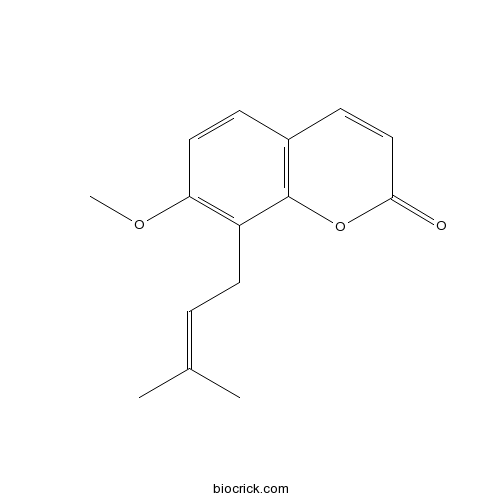
Osthol484-12-8
Instructions
-
BCN1061
Formononetin485-72-3
Instructions

-
BCN5926
Ononin486-62-4
Instructions

-
BCN5945
Isoliquiritin5041-81-6
Instructions

-
BCN5616
Oleanolic acid508-02-1
Instructions

-
BCN6663
Neoliquiritin5088-75-5
Instructions

-
BCN5024
Fisetin528-48-3
Instructions

-
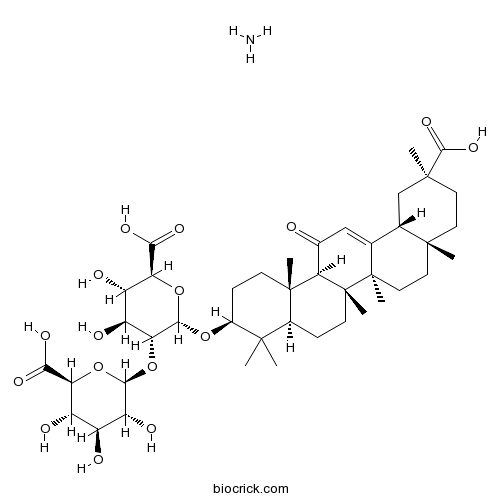
BCN5943
Glycyrrhizic acid ammonium salt53956-04-0
Instructions
-
BCN5730
Galangin548-83-4
Instructions

-
BCN5944
Liquiritin551-15-5
Instructions

-
BCN1209
Eriodictyol552-58-9
Instructions

-
BCN5891
Daidzin552-66-9
Instructions

-
BCN5946
Liquiritigenin578-86-9
Instructions

-
BCN6332
Licochalcone A58749-22-7
Instructions

-
BCN6333
Licochalcone B58749-23-8
Instructions

-
BCN1221
Glabridin59870-68-7
Instructions
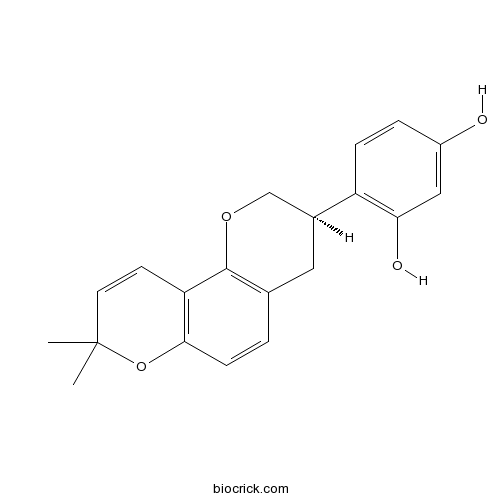
-
BCN2929
Licoisoflavone A66056-19-7
Instructions

-
BCN9061
(±)-Naringenin67604-48-2
Instructions
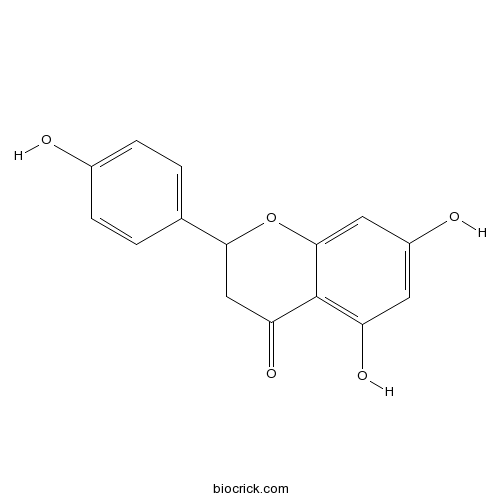
-
BCX1186
Neoisoliquiritigenin7014-39-3
Instructions

-
BCN4294
2,3-Dehydrokievitone74161-25-4
Instructions

-
BCN4512
Isoliquiritigenin961-29-5
Instructions
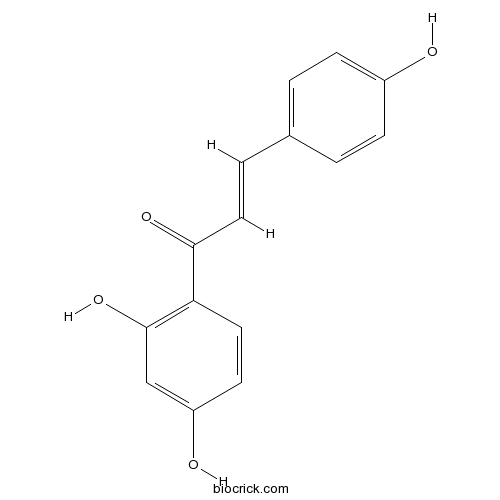
Prenylated Flavonoids from Roots of Glycyrrhiza uralensis Induce Differentiation of B16-F10 Melanoma Cells.[Pubmed: 30115883]
None
[Complex network analysis of law on Chinese herbal drugs intervention on radiation induced lung injury].[Pubmed: 30111064]
To mainly analyze the prescription rules of Chinese herbal drugs for radiation induced lung injury, optimize the prescriptions, and provide a reference for the clinical treatment of radiation induced lung injury. The major Chinese databases CNKI, CBM and Wanfang data were searched to obtain the literature on Chinese herbal drugs for radiation induced lung injury. BICOMS 2 software was used to extract and collect all Chinese herbal drugs information and generate the co-occurrence matrix; NetDraw and Gcluto software were then used to make network map and visualization matrix for analysis. A total of 552 articles (19 types and 304 Chinese herbal drugs) were included. Ophiopogon japonicus had the highest frequency (229 times), followed by Astragalus membranaceus(181 times), Glycyrrhiza uralensis (166 times), and Scutellaria baicalensis (150 times). After the classification of efficacy, deficiency-supplementing medicinal (69 kinds of Chinese herbs), heat-clearing medicine (51 kinds of Chinese herbs) and phlegm cough medicine (42 kinds of Chinese herbs) accounted for 53.29% of all the Chinese herbs, acting in the main position. After the prescription analysis for the top 25 herbal prescriptions, six main structures of common prescriptions were found for the treatment of radiation induced lung injury. There are many kinds of Chinese herbal drugs for the treatment of radiation induced lung injury in clinical application. In the future, researchers can mainly focus on Ophiopogon japonicus etc. as the main drugs, combine with other high-frequency Chinese herbal drugs found in this study, or directly refer to the main structures of commonly used prescriptions found in this analysis.
Anti-melanogenesis effect of dehydroglyasperin C through the downregulation of MITF via the reduction of intracellular cAMP and acceleration of ERK activation in B16F1 melanoma cells.[Pubmed: 30099299]
In mammals, UV radiation induces melanin synthesis in melanocyte for protecting their skin through the stimulation of α-melanocyte stimulating hormone (α-MSH) from keratinocytes. In this study, the inhibitory effects of dehydroglyasperin C (DGC), an useful component of Glycyrrhiza uralensis (G. uralensis), was investigated on melanogenesis induced by α-melanocyte stimulating hormone (α-MSH) and its mechanisms.
Isoliquiritigenin suppresses human melanoma growth by targeting miR-301b/LRIG1 signaling.[Pubmed: 30081934]
Isoliquiritigenin (ISL), a natural flavonoid isolated from the root of licorice (Glycyrrhiza uralensis), has shown various pharmacological properties including anti-oxidant, anti-inflammatory and anti-cancer activities. MicroRNAs (miRNAs), a class of small non-coding RNAs, have been reported as post-transcriptional regulators with altered expression levels in melanoma. This study aims to investigate the anti-melanoma effect of ISL and its potential mechanism.
Biological Effects Of Licochalcones.[Pubmed: 30049263]
Licorice root (Radix Glycyrrhizae) is a common perennial plants native to Mediterranean countries, central to southern Russia, Asia, Turkey, Iraq and Iran used in traditional Chinese medicine for centuries and licorice has been described as 'the grandfather of herbs' .The genus name Glycyrrhiza (family Leguminoseae) is derived from the ancient Greek words glycos (meaning sweet) and rhiza (meaning root). It consists of about 30 species, but most common ones are Glycyrrhiza glabra L., Glycyrrhiza uralensis Fisch and Glycyrrhiza Inflata. It is known that licorice root contains various chalcones which are an important class of natural products, precursors of flavonoids. Chemically, chalcones consist of open-chain flavonoids, wherein two aromatic rings are joined by a three-carbon α, β-unsaturated ketone, which represent the fundamental nucleus of the structure. They are classified according to chemical structures in Licochalcone A, B, C, D, E, F and G. This review aims to highlight all the in vitro and in vivo studies that have been conducted on the licochalcones, extracted from Glycyrrhiza species. The most important effects are: anti-inflammatory, antioxidant, anticancer, antimicrobial, antiviral, antiallergic, antidiabetic, hepatotoxic and osteogenic. Natural bioactive compounds are mostly value to implement the introduction of biologically active molecules from the bench (research) to the bedside (clinical practice). However, in a future perspective it is necessary to perform additional studies to confirm these biological effects.
Isoliquiritigenin attenuates glutamate-induced mitochondrial fission via calcineurin-mediated Drp1 dephosphorylation in HT22 hippocampal neuron cells.[Pubmed: 30048666]
Numerous studies suggest that glutamate toxicity is a major contributor to neuronal dysfunction and death in several neurodegenerative diseases. In our previous study, isoliquiritigenin (ISL) isolated from Glycyrrhiza uralensis showed neuroprotective effects against neuronal cell death mediated by intracellular reactive oxygen species (ROS) generation and loss of mitochondrial membrane potential. However, the mechanisms by which ISL protects against glutamate-induced oxidative stress are unknown. In the present study, we focused on the cellular and molecular mechanisms underlying the inhibition of ROS production and induction of mitochondrial dysfunction by ISL in glutamate-stimulated HT22 mouse hippocampal neuron cells. The results revealed that ISL inhibited glutamate-induced mitochondrial ROS production and decline of glutathione levels and ATP generation in HT22 cells. Interestingly, we discovered that ISL prevents glutamate-induced mitochondrial fission by inhibiting the dephosphorylation of Drp1 at the serine 637 residue, which is a regulatory factor of mitochondrial dynamics, and both a S637D mutation of Drp1, which resulted in a phosphorylation-mimetic form of Drp1 at Ser637, and mitochondria-targeted antioxidant Mito-TEMPO inhibited glutamate-induced mitochondrial fission. Furthermore, ISL also prevented the increase of intracellular calcium accompanied by activation of calcineurin, which is a key regulator of dephosphorylation of Drp1 (Ser637), in glutamate-treated HT22 cells. Taken together, our results demonstrated that ISL protects against glutamate-induced mitochondrial fission by inhibiting the increase of mitochondrial ROS and intracellular calcium, which are accompanied by dephosphorylation of Drp1 (Ser637), and consequently attenuates glutamate-induced neuronal cell death. Therefore, these findings suggest that ISL exhibits the potential for protection against glutamate toxicity. These results may contribute to the development of new drugs and novel strategies for the treatment of neurodegenerative disorders related to glutamate toxicity.
UGT73F17, a new glycosyltransferase from Glycyrrhiza uralensis, catalyzes the regiospecific glycosylation of pentacyclic triterpenoids.[Pubmed: 30010690]
The regiospecific glycosylation of pentacyclic triterpenoids by UGT73F17, a new glycosyltransferase from Glycyrrhiza uralensis, is highlighted. UGT73F17 exhibited strict substrate specificity toward the carboxyl group at C-30/C-29 of pentacyclic triterpenoids, and showed high promiscuity to sugar donors. UGT73F17 represents the first identified triterpenoid 30/29-O-glycosyltransferase, and could be used as an effective biocatalyst to synthesize glycosyl ester saponins.


